Difference between revisions of "Glycerol" - New World Encyclopedia
(claim, fix, edits) |
|||
| (20 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{Copyedited}} |
| − | {{ | ||
<!-- Here is a table of data; skip past it to edit the text. —> | <!-- Here is a table of data; skip past it to edit the text. —> | ||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
| Line 6: | Line 5: | ||
|- | |- | ||
| − | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:Glycerine chemical structure.png|200px|Glycerol]]<br>[[Image:Glycerol-3D-balls.png|200px|Ball-and-stick model of glycerol]]<br>[[Image:Glycerol-3D-vdW.png|200px|Space-filling model of glycerol]] | + | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:Glycerine chemical structure.png|200px|Glycerol]]<br/>[[Image:Glycerol-3D-balls.png|200px|Ball-and-stick model of glycerol]]<br/>[[Image:Glycerol-3D-vdW.png|200px|Space-filling model of glycerol]] |
|- | |- | ||
| [[IUPAC nomenclature|Chemical name]] | | [[IUPAC nomenclature|Chemical name]] | ||
| Line 24: | Line 23: | ||
|- | |- | ||
| [[Harmonized System number|HS number]] | | [[Harmonized System number|HS number]] | ||
| − | | Crude: 1520.00.00<br>Pure: 2905.45.00 | + | | Crude: 1520.00.00<br/>Pure: 2905.45.00 |
|- | |- | ||
| [[Density]] | | [[Density]] | ||
| Line 53: | Line 52: | ||
|- | |- | ||
| [[Glycerol (data page)#Thermodynamic properties|Thermodynamic data]] | | [[Glycerol (data page)#Thermodynamic properties|Thermodynamic data]] | ||
| − | | Phase behavior<br>Solid, liquid, gas | + | | Phase behavior<br/>Solid, liquid, gas |
|- | |- | ||
| [[Glycerol (data page)#Spectral data|Spectral data]] | | [[Glycerol (data page)#Spectral data|Spectral data]] | ||
| Line 62: | Line 61: | ||
|} | |} | ||
| − | '''Glycerol''' is a [[ | + | '''Glycerol''', also known as '''glycerin''' or '''glycerine''', is a [[sugar alcohol]]. Its [[chemical formula|formula]] may be written as C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>. It is a colorless, odorless, [[Viscosity|viscous]], sweet-tasting liquid that is soluble in water and low in toxicity. It is found in nature in the form of its [[ester]]s, which are known as '''glycerides'''. The glycerides are fundamental constituents of [[lipid]]s. |
| + | {{toc}} | ||
| + | Glycerol has numerous uses. For instance, it is added to pharmaceutical formulations as a means of providing [[lubrication]] and as a [[humectant]] (water-absorbing substance). It is a constituent of [[cough syrup]]s, elixirs, [[expectorant]]s, and [[suppository|suppositories]]. It is an ingredient in [[toothpaste]], [[mouthwash]], [[soap]]s, shaving cream, and various [[skin care]] and [[hair care]] products. It is added to various foods as a [[solvent]] for certain flavors; humectant and softening agent in [[candy]] and [[cakes]]; and as a preservative. It is used in the manufacture of [[paper]], various packaging materials, and [[nitroglycerin]]. It is also a softener of yarn and fabric. | ||
| + | |||
| + | == Notable characteristics == | ||
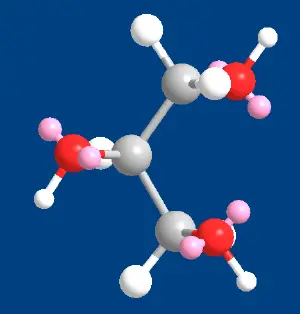
| + | [[Image:Glycerol-3DModel.png|thumb|left|In this 3D model of a glycerol molecule, the carbon atoms are shown as gray spheres, the oxygen atoms are red, and the hydrogen atoms are white. Each oxygen atom has a "lone" (unshared) electron pair, and these electrons are shown in pink. (Model is not to scale.)]] | ||
| + | |||
| + | Each glycerol molecule has a three-carbon chain, with a [[hydroxyl group]] (OH) attached to each carbon atom. To indicate this arrangement, its chemical formula may be written as HOCH<sub>2</sub>CH(OH)CH<sub>2</sub>OH. The hydroxyl groups are responsible for making the substance highly soluble in [[water]] and [[Hygroscopy|hygroscopic]]. (A hygroscopic substance is one that attracts water molecules from the surrounding environment.) It has only slight solubility in organic [[solvent]]s such as ethyl acetate and diethyl [[ether]], and it does not dissolve in [[hydrocarbon]]s. Its melting point is 18 °C (64.4 °F), and its boiling point is 290 °C (554 °F). | ||
==Synthesis== | ==Synthesis== | ||
| − | [[Image: | + | [[Image:Saponificación.svg|thumb|250px|Saponification of a [[lipid]] with [[potassium hydroxide]] produces the potassium salt of the lipid and glycerol.]] |
| − | + | ||
| − | Until recently, synthetic glycerol was | + | Until recently, synthetic glycerol was manufactured on an industrial scale mainly from [[epichlorohydrin]], but this process is no longer economical. Two major methods of producing glycerol from natural products are [[saponification]] and [[transesterification]]. |
| − | + | * Saponification: When an alkali (such as sodium hydroxide or potassium hydroxide) is reacted with a fat or oil, it forms soap (the salt of the lipid) and glycerol. | |
| − | + | * Transesterification: When a glyceride is reacted with an [[alcohol]], in the presence of an acid or base as catalyst, a new ester is formed and glycerol is released as a by-product. | |
| − | * | ||
| − | |||
| − | |||
| − | |||
| − | * | ||
| − | |||
| − | + | Glycerol is a 10 percent by-product of biodiesel manufacture, via the transesterification of vegetable oils. This has led to a glut of crude glycerol on the market. This crude glycerol (typically containing 20 percent water and residual esterification catalyst) can then be refined to a purified form. At the same time, a great deal of research is being conducted to try to make value-added molecules from glycerol, as an alternative to [[incineration]]. One such program to add value to this glut of glycerol is the U.K.-based initiative called the Glycerol Challenge.<ref>[http://www.theglycerolchallenge.org The Glycerol Challenge]. Retrieved September 19, 2007.</ref> Some potential uses for glycerol include its conversion to [[propylene glycol]],<ref>[http://www.dow.com/propyleneglycol/news/20070315b.htm Propylene Glycol]. Dow Chemical. Retrieved September 14, 2007.</ref> [[acrolein]],<ref>L. Ott, et al. "The catalytic dehydration of glycerol in sub- and supercritical water: a new chemical process for acrolein production." ''Green Chemistry'' 8 (2) (2006) :214-220.</ref><ref>M. Watanabe, et al. "Acrolein synthesis from glycerol in hot-compressed water." ''Bioresource technology'' 98 (2007) :1285-1290.</ref> [[ethanol]],<ref>[http://www.sciencedaily.com/upi/index.php?feed=Science&article=UPI-1-20070625-13174400-bc-us-glycerin.xml Technology turns glycerin into ethanol]. Science Daily. Retrieved September 14, 2007.</ref> and [[epichlorhydrin]] (a raw material for [[epoxy resins]]).<ref>[http://epoxy.dow.com/epoxy/news/2007/20070326b.htm Dow Epoxy]. Dow Chemical. Retrieved September 14, 2007.</ref> It could also be used to produce hydrogen gas or [[citric acid]]. | |
| − | Glycerol is a | ||
| − | + | ==Involvement in metabolic pathways== | |
| + | |||
| + | Glycerol is a precursor for synthesis of [[triacylglycerol]]s and [[phospholipid]]s in the liver and adipose tissue. When the body uses stored fat as a source of energy, glycerol and [[fatty acid]]s are released into the bloodstream. The glycerol component can be converted to [[glucose]] by the [[liver]] and provides energy for cellular metabolism. | ||
| + | |||
| + | Depending on physiological conditions, glycerol enters the pathway of [[glycolysis]] (breakdown of glucose and other sugars) or [[gluconeogenesis]] (glucose formation). Prior to entering either pathway, glycerol is converted to the intermediate known as [[glyceraldehyde 3-phosphate]], in the following steps: | ||
{|class="toccolours" border="0" style="font-size:90%" align="center" | {|class="toccolours" border="0" style="font-size:90%" align="center" | ||
|- | |- | ||
| Line 96: | Line 99: | ||
| rowspan="3" align="center" | [[image:Glycerol-3-phosphate.png|100px]] | | rowspan="3" align="center" | [[image:Glycerol-3-phosphate.png|100px]] | ||
| valign="bottom" align="center" | NAD<sup>+</sup> | | valign="bottom" align="center" | NAD<sup>+</sup> | ||
| − | | valign="bottom" align="center" | NADH<br>+ H<sup>+</sup> | + | | valign="bottom" align="center" | NADH<br/>+ H<sup>+</sup> |
| rowspan="3" align="center" | [[image:DHAP.png|125px]] | | rowspan="3" align="center" | [[image:DHAP.png|125px]] | ||
| | | | ||
| Line 102: | Line 105: | ||
| rowspan="2" align="center" | [[image:G3P-2D-skeletal.png|150px]] | | rowspan="2" align="center" | [[image:G3P-2D-skeletal.png|150px]] | ||
|- | |- | ||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| valign="top" | | | valign="top" | | ||
| valign="top" | | | valign="top" | | ||
| − | | valign="top" align="center" | NADH<br>+ H<sup>+</sup> | + | | valign="top" align="center" | NADH<br/>+ H<sup>+</sup> |
| valign="top" align="center" | NAD<sup>+</sup> | | valign="top" align="center" | NAD<sup>+</sup> | ||
| valign="top" | | | valign="top" | | ||
| Line 117: | Line 117: | ||
==Applications== | ==Applications== | ||
| + | |||
| + | Glycerol is useful for numerous applications. Some of them are listed below. | ||
| + | |||
===Medicine and pharmaceutical technology=== | ===Medicine and pharmaceutical technology=== | ||
| − | * | + | |
| − | * | + | * Glycerol is used in medical and pharmaceutical preparations, mainly as a means of improving smoothness, providing [[lubrication]], and as a [[humectant]] (hygroscopic substance). It may also be used to lower intracranial and intraocular pressure. |
| − | * | + | * It acts as a [[laxative]] when introduced into the rectum in [[suppository]] or liquid ([[enema]]) form. |
| − | Walter S. Long | + | * It is used in [[cough syrup]]s, elixirs, and [[expectorant]]s. |
| − | + | * In the production of [[tincture]]s, glycerol (at 10 percent concentration) is used to prevent tannins from precipitating in ethanol extracts of plants. | |
| − | + | * It may be used as a substitute for alcohol, as a solvent that will create a therapeutic herbal extraction, but is less extractive and is approximately 30% less able to be absorbed by the body. [[Fluid extract]] manufacturers often extract herbs in hot water before adding glycerin to make [[glycerites]].<ref>Walter S. Long, "The Composition of Commercial Fruit Extracts." 1916, 1917. ''Transactions of the Kansas Academy of Science (1903-)'' 28:157-161.</ref><ref>[http://www.newhope.com/nutritionsciencenews/NSN_backs/Apr_99/backtalk.cfm Does Alcohol Belong In Herbal Tinctures?]. Backtalk: ''Nutrition Science News'' (April 1999) Retrieved September 14, 2007.</ref> | |
| − | |||
===Personal care=== | ===Personal care=== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | It was once believed that when used as an emollient, glycerol should never be applied undiluted to the skin. | + | * Glycerol serves as an [[emollient]], [[humectant]], solvent, and [[personal lubricant|lubricant]] in personal care products. |
| + | * It competes with [[sorbitol]], although glycerol is considered to have better taste and higher solubility. | ||
| + | * It is used in [[toothpaste]], [[mouthwash]], [[skin care]] products, shaving cream, [[hair care]] products and [[soap]]s. | ||
| + | **Glycerol is a component of glycerol [[soap]], which is made from [[denatured alcohol]], glycerol, sodium castorate (from [[Castor bean|castor]]), [[sodium cocoate]], [[sodium tallowate]], [[sucrose]], water and [[parfum]] ([[fragrance]]). Sometimes one adds [[sodium laureth sulfate]]. This kind of soap is used by people with sensitive, easily irritated [[skin]] because it prevents skin dryness with its moisturizing]] properties. It is possible to make glycerol soap at home. | ||
| + | |||
| + | It was once believed that when used as an emollient, glycerol should never be applied undiluted to the skin. It was thought that just as glycerol draws moisture out of the air to moisten the skin, it would draw moisture out of the skin if it were too concentrated. This fear has proven to be unfounded. | ||
===Foods and beverages=== | ===Foods and beverages=== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Glycerol | + | * Glycerol serves as a [[humectant]], solvent and sweetener, and it may help preserve foods. |
| + | * It is a solvent for flavors (such as [[vanilla]]) and [[food coloring]]. | ||
| + | * [[Humectant]] and softening agent in [[candy]], [[cakes]], and casings for [[meat]]s and [[cheeses]]. | ||
| + | * It is used in the manufacture of mono- and di-glycerides, which act as [[emulsifier]]s. | ||
| + | * Used in manufacture of polyglycerol [[ester]]s going into [[shortening]]s and [[margarine]]. | ||
| + | * Used as a filler in low-fat food products (including [[cookie]]s). | ||
| + | * Used as [[thickening agent]] in [[liqueur]]s. | ||
| + | * Produced when [[butter]] becomes [[Rancidification|rancid]]. | ||
| − | + | Glycerol has approximately 27 calories per teaspoon and is 60 percent as sweet as [[sucrose]]. Although it has about the same [[food energy]] as table sugar, it does not raise blood sugar levels, nor does it feed the bacteria that form plaques and cause dental cavities. Glycerol should not be consumed undiluted, as unhydrated glycerol will draw water from tissues, causing blistering in the mouth and gastric distress. As a food additive, glycerol is also known as [[E number]] E422. | |
| − | Glycerol is | ||
===Polyether polyols=== | ===Polyether polyols=== | ||
| + | |||
*One of the major raw materials for the manufacture of [[polyol]]s for flexible foams, and to a lesser extent rigid [[polyurethane]] foams | *One of the major raw materials for the manufacture of [[polyol]]s for flexible foams, and to a lesser extent rigid [[polyurethane]] foams | ||
*Glycerol is the initiator to which propylene oxide/ethylene oxide is added | *Glycerol is the initiator to which propylene oxide/ethylene oxide is added | ||
===Alkyd resins (plastics) and cellophane=== | ===Alkyd resins (plastics) and cellophane=== | ||
| − | * | + | |
| − | * | + | * When reacted with a dibasic acid (such as phthalic acid) it forms a class of products called ''alkyd resins,'' which are used in surface coatings and paints. |
| − | * | + | * It is a softener and plasticizer (such as in [[cellophane]]), imparting flexibility, pliability, and toughness. |
| − | + | * It is used in meat casings, collagen casings (medical applications), and nonmeat packaging. | |
===Absolute alcohol=== | ===Absolute alcohol=== | ||
| − | * | + | |
| + | * A process to produce [[absolute alcohol]] involves dehydration of alcohol using glycerol. | ||
===Other applications=== | ===Other applications=== | ||
| − | *Manufacture of paper as a [[plasticizer]], [[ | + | |
| − | *Used in lubricating, sizing and softening of yarn and fabric | + | * Manufacture of paper as a [[plasticizer]], humectant, and [[lubricant]]. |
| − | *Used in de-/anti-icing fluids, as in [[vitrification]] of blood cells for storage in [[liquid nitrogen]] | + | * Manufacture of [[nitroglycerin]], an essential ingredient of smokeless gunpowder and various munitions. Processes to produce synthetic glycerin were national defense priorities in the days leading up to World War II. |
| − | *Patent applications have been filed for detergent [[Water softener|softeners]] and [[surfactants]] based on glycerol (i.e., alkyl glyceryl [[ether]]s) instead of [[quaternary ammonium cation|quaternary ammonium]] compounds. | + | * Used in lubricating, sizing, and softening of yarn and fabric. |
| − | * | + | * Used in de-/anti-icing fluids, as in [[vitrification]] of blood cells for storage in [[liquid nitrogen]]. |
| − | + | * Patent applications have been filed for detergent [[Water softener|softeners]] and [[surfactants]] based on glycerol (i.e., alkyl glyceryl [[ether]]s) instead of [[quaternary ammonium cation|quaternary ammonium]] compounds. | |
| − | *Often used in the preparation of lichen for use in model scenery and dioramas | + | * One way to preserve leaves is to submerge them in a solution of glycerol and water.<ref>Use a mixture of one part glycerol to two parts water. Place the mixture in a flat pan, and totally submerge the leaves (by weighing them down) in a single layer in the liquid. In two to six days, they should have absorbed the liquid and be soft and pliable. Remove them from the pan and wipe off all the liquid with a soft cloth. Done correctly, the leaves will remain soft and pliable indefinitely.</ref> |
| − | *Can be added to | + | * Often used in the preparation of lichen for use in model scenery and dioramas |
| + | * Can be added to a solution of water and soap to increase the solution's ability to generate long-lasting [[soap bubble]]s. | ||
*Used as an antifreeze or a [[cryoprotectant]] in [[cryogenic]] process. | *Used as an antifreeze or a [[cryoprotectant]] in [[cryogenic]] process. | ||
| − | *Used in [[fog machine]] fluids | + | *Used in [[fog machine]] fluids. |
| − | + | *Counteracts [[phenol]] burns. | |
| − | *Counteracts [[phenol]] burns | ||
*Now that biodiesel production likely will produce large quantities of co-product glycerine (about 0.1 lb of glycerine per lb of biodiesel), processes are being announced to manufacture [[propylene glycol]] and [[epichlorohydrin]], traditionally [[propylene]] derivatives, from glycerine. | *Now that biodiesel production likely will produce large quantities of co-product glycerine (about 0.1 lb of glycerine per lb of biodiesel), processes are being announced to manufacture [[propylene glycol]] and [[epichlorohydrin]], traditionally [[propylene]] derivatives, from glycerine. | ||
| − | *A process has been announced to produce [[ethanol]] through the metabolic action of [[E. coli]]<ref>http://www.sciencedaily.com/releases/2007/06/070626115246.htm</ref>. | + | *A process has been announced to produce [[ethanol]] through the metabolic action of [[E. coli]] on glycerin.<ref>[http://www.sciencedaily.com/releases/2007/06/070626115246.htm Engineers Find Way To Make Ethanol, Valuable Chemicals From Waste Glycerin]. Science Daily. Retrieved September 14, 2007.</ref>. |
*Used by some endurance athletes to counteract dehydration by "glycerol loading" before an event. | *Used by some endurance athletes to counteract dehydration by "glycerol loading" before an event. | ||
| − | *Used to preserve bacteria at - | + | *Used to preserve bacteria at sub-freezing temperatures (prevents lysing of cells). |
| − | + | *Used in the conservation of waterlogged organic objects (such as leather and wood) to stabilize before freeze-drying treatment. | |
| − | |||
| − | |||
| − | *Used in the conservation of waterlogged organic objects (such as leather and wood) to | ||
*Used in ink for desktop printers as a viscosity controller and stabilizer. | *Used in ink for desktop printers as a viscosity controller and stabilizer. | ||
| + | * It is a ([[prochiral]]) building block in [[organic synthesis]]. | ||
==Danger of contamination with diethylene glycol== | ==Danger of contamination with diethylene glycol== | ||
| − | On May 4, 2007, the | + | On May 4, 2007, the U.S. [[Food and Drug Administration]] advised all U.S. makers of medicines to test all batches of glycerine for the toxic [[diethylene glycol]].<ref>[http://www.fda.gov/bbs/topics/NEWS/2007/NEW01628.html FDA Advises Manufacturers to Test Glycerin for Possible Contamination.] U.S. Food and Drug Administration. Retrieved September 14, 2007.</ref> This follows an occurrence of 100 fatal poisonings in [[Panama]] resulting from a Chinese factory deliberately falsifying records in order to export the cheaper diethylene glycol as the more expensive glycerol. Glycerine and diethylene glycol are similar in appearance, smell, and taste. The U.S. [[Federal Food, Drug, and Cosmetic Act]] was passed following the 1937 "[[Elixir Sulfanilamide]]" incident of poisoning caused by diethylene glycol contamination of medicine. |
==See also== | ==See also== | ||
| Line 198: | Line 199: | ||
==References== | ==References== | ||
| − | * | + | * McMurry, John. 2004. ''Organic Chemistry,'' 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052. |
| + | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry,'' 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2. | ||
| + | * Solomons, T.W. Graham, and Fryhle, Craig B. 2004. ''Organic Chemistry,'' 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998. | ||
==External links== | ==External links== | ||
| − | *[http://www. | + | All links retrieved June 23, 2017. |
| − | *[http://journeytoforever.org/biofuel_library/Mariller.html Absolute alcohol using glycerol] | + | * [http://www.health.gov/dietaryguidelines/dga2005/report/HTML/G1_Glossary.htm Nutrition and Your Health: Dietary Guidelines for Americans.] ''U.S. Department of Agriculture''. |
| − | + | *[http://journeytoforever.org/biofuel_library/Mariller.html Absolute alcohol using glycerol]. | |
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Latest revision as of 18:22, 30 August 2021
| Glycerol | |
|---|---|
| |
| Chemical name | Propane-1,2,3-triol |
| Other names | glycerin glycerine propane-1,2,3-triol 1,2,3-propanetriol 1,2,3-trihydroxypropane glyceritol glycyl alcohol |
| Chemical formula | C3H5(OH)3 |
| Molecular mass | 92.09382 g/mol |
| CAS number | [56-81-5] |
| HS number | Crude: 1520.00.00 Pure: 2905.45.00 |
| Density | 1.261 g/cm³ |
| Viscosity | 1.5 Pa.s |
| Melting point | 18 °C (64.4°F) |
| Boiling point | 290 °C (554°F) |
| Food energy | 4.32 kcal/g |
| SMILES | OCC(O)CO |
| Flash Point | 160 °C (closed cup) |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behavior Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Disclaimer and references | |
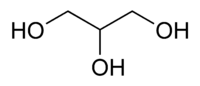
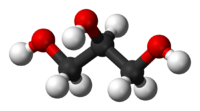
Glycerol, also known as glycerin or glycerine, is a sugar alcohol. Its formula may be written as C3H8O3. It is a colorless, odorless, viscous, sweet-tasting liquid that is soluble in water and low in toxicity. It is found in nature in the form of its esters, which are known as glycerides. The glycerides are fundamental constituents of lipids.
Glycerol has numerous uses. For instance, it is added to pharmaceutical formulations as a means of providing lubrication and as a humectant (water-absorbing substance). It is a constituent of cough syrups, elixirs, expectorants, and suppositories. It is an ingredient in toothpaste, mouthwash, soaps, shaving cream, and various skin care and hair care products. It is added to various foods as a solvent for certain flavors; humectant and softening agent in candy and cakes; and as a preservative. It is used in the manufacture of paper, various packaging materials, and nitroglycerin. It is also a softener of yarn and fabric.
Notable characteristics
Each glycerol molecule has a three-carbon chain, with a hydroxyl group (OH) attached to each carbon atom. To indicate this arrangement, its chemical formula may be written as HOCH2CH(OH)CH2OH. The hydroxyl groups are responsible for making the substance highly soluble in water and hygroscopic. (A hygroscopic substance is one that attracts water molecules from the surrounding environment.) It has only slight solubility in organic solvents such as ethyl acetate and diethyl ether, and it does not dissolve in hydrocarbons. Its melting point is 18 °C (64.4 °F), and its boiling point is 290 °C (554 °F).
Synthesis
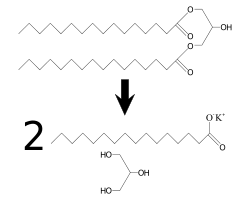
Until recently, synthetic glycerol was manufactured on an industrial scale mainly from epichlorohydrin, but this process is no longer economical. Two major methods of producing glycerol from natural products are saponification and transesterification.
- Saponification: When an alkali (such as sodium hydroxide or potassium hydroxide) is reacted with a fat or oil, it forms soap (the salt of the lipid) and glycerol.
- Transesterification: When a glyceride is reacted with an alcohol, in the presence of an acid or base as catalyst, a new ester is formed and glycerol is released as a by-product.
Glycerol is a 10 percent by-product of biodiesel manufacture, via the transesterification of vegetable oils. This has led to a glut of crude glycerol on the market. This crude glycerol (typically containing 20 percent water and residual esterification catalyst) can then be refined to a purified form. At the same time, a great deal of research is being conducted to try to make value-added molecules from glycerol, as an alternative to incineration. One such program to add value to this glut of glycerol is the U.K.-based initiative called the Glycerol Challenge.[1] Some potential uses for glycerol include its conversion to propylene glycol,[2] acrolein,[3][4] ethanol,[5] and epichlorhydrin (a raw material for epoxy resins).[6] It could also be used to produce hydrogen gas or citric acid.
Involvement in metabolic pathways
Glycerol is a precursor for synthesis of triacylglycerols and phospholipids in the liver and adipose tissue. When the body uses stored fat as a source of energy, glycerol and fatty acids are released into the bloodstream. The glycerol component can be converted to glucose by the liver and provides energy for cellular metabolism.
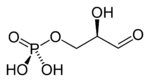
Depending on physiological conditions, glycerol enters the pathway of glycolysis (breakdown of glucose and other sugars) or gluconeogenesis (glucose formation). Prior to entering either pathway, glycerol is converted to the intermediate known as glyceraldehyde 3-phosphate, in the following steps:
| Glycerol | Glycerol kinase | Glycerol-3-phosphate | Glycerol-3-phosphate dehydrogenase | Dihydroxyacetone phosphate | Triosephosphate isomerase | Glyceraldehyde 3-phosphate | |||
| ATP | ADP | NAD+ | NADH + H+ |
| |||||
| NADH + H+ |
NAD+ | ||||||||
The enzyme glycerol kinase is present only in the liver. In adipose tissue, glycerol 3-phosphate is obtained from dihydroxyacetone phosphate (DHAP) with the enzyme glycerol-3-phosphate dehydrogenase.
Applications
Glycerol is useful for numerous applications. Some of them are listed below.
Medicine and pharmaceutical technology
- Glycerol is used in medical and pharmaceutical preparations, mainly as a means of improving smoothness, providing lubrication, and as a humectant (hygroscopic substance). It may also be used to lower intracranial and intraocular pressure.
- It acts as a laxative when introduced into the rectum in suppository or liquid (enema) form.
- It is used in cough syrups, elixirs, and expectorants.
- In the production of tinctures, glycerol (at 10 percent concentration) is used to prevent tannins from precipitating in ethanol extracts of plants.
- It may be used as a substitute for alcohol, as a solvent that will create a therapeutic herbal extraction, but is less extractive and is approximately 30% less able to be absorbed by the body. Fluid extract manufacturers often extract herbs in hot water before adding glycerin to make glycerites.[7][8]
Personal care
- Glycerol serves as an emollient, humectant, solvent, and lubricant in personal care products.
- It competes with sorbitol, although glycerol is considered to have better taste and higher solubility.
- It is used in toothpaste, mouthwash, skin care products, shaving cream, hair care products and soaps.
- Glycerol is a component of glycerol soap, which is made from denatured alcohol, glycerol, sodium castorate (from castor), sodium cocoate, sodium tallowate, sucrose, water and parfum (fragrance). Sometimes one adds sodium laureth sulfate. This kind of soap is used by people with sensitive, easily irritated skin because it prevents skin dryness with its moisturizing]] properties. It is possible to make glycerol soap at home.
It was once believed that when used as an emollient, glycerol should never be applied undiluted to the skin. It was thought that just as glycerol draws moisture out of the air to moisten the skin, it would draw moisture out of the skin if it were too concentrated. This fear has proven to be unfounded.
Foods and beverages
- Glycerol serves as a humectant, solvent and sweetener, and it may help preserve foods.
- It is a solvent for flavors (such as vanilla) and food coloring.
- Humectant and softening agent in candy, cakes, and casings for meats and cheeses.
- It is used in the manufacture of mono- and di-glycerides, which act as emulsifiers.
- Used in manufacture of polyglycerol esters going into shortenings and margarine.
- Used as a filler in low-fat food products (including cookies).
- Used as thickening agent in liqueurs.
- Produced when butter becomes rancid.
Glycerol has approximately 27 calories per teaspoon and is 60 percent as sweet as sucrose. Although it has about the same food energy as table sugar, it does not raise blood sugar levels, nor does it feed the bacteria that form plaques and cause dental cavities. Glycerol should not be consumed undiluted, as unhydrated glycerol will draw water from tissues, causing blistering in the mouth and gastric distress. As a food additive, glycerol is also known as E number E422.
Polyether polyols
- One of the major raw materials for the manufacture of polyols for flexible foams, and to a lesser extent rigid polyurethane foams
- Glycerol is the initiator to which propylene oxide/ethylene oxide is added
Alkyd resins (plastics) and cellophane
- When reacted with a dibasic acid (such as phthalic acid) it forms a class of products called alkyd resins, which are used in surface coatings and paints.
- It is a softener and plasticizer (such as in cellophane), imparting flexibility, pliability, and toughness.
- It is used in meat casings, collagen casings (medical applications), and nonmeat packaging.
Absolute alcohol
- A process to produce absolute alcohol involves dehydration of alcohol using glycerol.
Other applications
- Manufacture of paper as a plasticizer, humectant, and lubricant.
- Manufacture of nitroglycerin, an essential ingredient of smokeless gunpowder and various munitions. Processes to produce synthetic glycerin were national defense priorities in the days leading up to World War II.
- Used in lubricating, sizing, and softening of yarn and fabric.
- Used in de-/anti-icing fluids, as in vitrification of blood cells for storage in liquid nitrogen.
- Patent applications have been filed for detergent softeners and surfactants based on glycerol (i.e., alkyl glyceryl ethers) instead of quaternary ammonium compounds.
- One way to preserve leaves is to submerge them in a solution of glycerol and water.[9]
- Often used in the preparation of lichen for use in model scenery and dioramas
- Can be added to a solution of water and soap to increase the solution's ability to generate long-lasting soap bubbles.
- Used as an antifreeze or a cryoprotectant in cryogenic process.
- Used in fog machine fluids.
- Counteracts phenol burns.
- Now that biodiesel production likely will produce large quantities of co-product glycerine (about 0.1 lb of glycerine per lb of biodiesel), processes are being announced to manufacture propylene glycol and epichlorohydrin, traditionally propylene derivatives, from glycerine.
- A process has been announced to produce ethanol through the metabolic action of E. coli on glycerin.[10].
- Used by some endurance athletes to counteract dehydration by "glycerol loading" before an event.
- Used to preserve bacteria at sub-freezing temperatures (prevents lysing of cells).
- Used in the conservation of waterlogged organic objects (such as leather and wood) to stabilize before freeze-drying treatment.
- Used in ink for desktop printers as a viscosity controller and stabilizer.
- It is a (prochiral) building block in organic synthesis.
Danger of contamination with diethylene glycol
On May 4, 2007, the U.S. Food and Drug Administration advised all U.S. makers of medicines to test all batches of glycerine for the toxic diethylene glycol.[11] This follows an occurrence of 100 fatal poisonings in Panama resulting from a Chinese factory deliberately falsifying records in order to export the cheaper diethylene glycol as the more expensive glycerol. Glycerine and diethylene glycol are similar in appearance, smell, and taste. The U.S. Federal Food, Drug, and Cosmetic Act was passed following the 1937 "Elixir Sulfanilamide" incident of poisoning caused by diethylene glycol contamination of medicine.
See also
Notes
- ↑ The Glycerol Challenge. Retrieved September 19, 2007.
- ↑ Propylene Glycol. Dow Chemical. Retrieved September 14, 2007.
- ↑ L. Ott, et al. "The catalytic dehydration of glycerol in sub- and supercritical water: a new chemical process for acrolein production." Green Chemistry 8 (2) (2006) :214-220.
- ↑ M. Watanabe, et al. "Acrolein synthesis from glycerol in hot-compressed water." Bioresource technology 98 (2007) :1285-1290.
- ↑ Technology turns glycerin into ethanol. Science Daily. Retrieved September 14, 2007.
- ↑ Dow Epoxy. Dow Chemical. Retrieved September 14, 2007.
- ↑ Walter S. Long, "The Composition of Commercial Fruit Extracts." 1916, 1917. Transactions of the Kansas Academy of Science (1903-) 28:157-161.
- ↑ Does Alcohol Belong In Herbal Tinctures?. Backtalk: Nutrition Science News (April 1999) Retrieved September 14, 2007.
- ↑ Use a mixture of one part glycerol to two parts water. Place the mixture in a flat pan, and totally submerge the leaves (by weighing them down) in a single layer in the liquid. In two to six days, they should have absorbed the liquid and be soft and pliable. Remove them from the pan and wipe off all the liquid with a soft cloth. Done correctly, the leaves will remain soft and pliable indefinitely.
- ↑ Engineers Find Way To Make Ethanol, Valuable Chemicals From Waste Glycerin. Science Daily. Retrieved September 14, 2007.
- ↑ FDA Advises Manufacturers to Test Glycerin for Possible Contamination. U.S. Food and Drug Administration. Retrieved September 14, 2007.
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2.
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
External links
All links retrieved June 23, 2017.
- Nutrition and Your Health: Dietary Guidelines for Americans. U.S. Department of Agriculture.
- Absolute alcohol using glycerol.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.