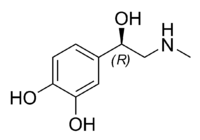
Epinephrine
| |
Epinephrine
| |
| Systematic name | |
| IUPAC name (R)-4-(1-hydroxy- 2-(methylamino)ethyl)benzene-1,2-diol | |
| Identifiers | |
| CAS number | 51-43-4 |
| ATC code | A01AD01 |
| PubChem | 838.1 |
| DrugBank | APRD00450 |
| Chemical data | |
| Formula | C9H13NO3 |
| Mol. weight | 183.204 g/mol |
| Pharmacokinetic data | |
| Bioavailability | Nil (oral) |
| Metabolism | adrenergic synapse (MAO and COMT) |
| Half life | 2 minutes |
| Excretion | n/a |
| Therapeutic considerations | |
| Pregnancy cat. | ? |
| Legal status | ? |
| Routes | IV, IM, endotracheal |
Epinephrine or adrenaline, sometimes spelled "epinephrin" or "adrenalin" respectively, is a hormone that is secreted principally by the adrenal medulla in response to physical or mental stress. Known as the "flight or fight response," this hormone stimulates the sympathetic nervous system, increasing heart rate, force of heart contractions, blood pressure, and breakdown (hydrolysis) of glycogen inot glucose, and elevates blood glucose levels. In short, it prepares the body for action in perceived emergency situations. The hormone boosts the supply of oxygen and energy-giving glucose to the brain and muscles; some bodily processes not vital to the response are suppressed.
Epinephrine is one of two main hormones excreted by the adrenal medulla, the other being norepinephrine.
Chemistry and overview
Epinephrine is a hormone and phenethylamine. It is a catecholamine, a sympathomimetic monoamine derived from the amino acids phenylalanine and tyrosine. The Latin roots ad-+renes and the Greek roots epi-+nephros both literally mean "on/to the kidney" (referring to the adrenal gland, which sits atop the kidneys and secretes epinephrine). Epinephrine is sometimes shortened to epi in medical jargon.
Catecholamines are chemical compounds derived from the amino acid tyrosine. Some of them are biogenic amines. Catecholamines are water soluble and are 50% bound to plasma proteins, so they circulate in the bloodstream. The most abundant catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline) and dopamine. Catecholamines as hormones are released by the adrenal glands in situations of stress such as psychological stress or low blood sugar levels
The adrenal glands are chiefly responsible for regulating the stress response through the synthesis of corticosteroids and catecholamines, including cortisol and adrenaline.
The adrenal gland is separated into two distinct structures, the adrenal medulla and the adrenal cortex. Both structures receive regulatory input from the nervous system.
The adrenal medulla takes up less than a quarter of the mass of the adrenal gland and is made of modified sympathetic ganglia. It is the body's main source of the catecholamine hormones adrenaline (epinephrine) and noradrenaline (norepinephrine). The adrenal medulla is at the center of the adrenal gland and is surrounded by the adrenal cortex, which forms the remaining three-quarters of the adrenal gland.
History
In May 1886, William Bates reported the discovery of a substance produced by the adrenal gland in the New York Medical Journal. Epinephrine was isolated and identified in 1895 by Napoleon Cybulski, a Polish physiologist. The discovery was repeated in 1897 by John Jacob Abel.[1]
Jokichi Takamine, a Japanese chemist, discovered the same hormone in 1900, without knowing about the previous discovery.[2][3]
It was first artificially synthesized in 1904 by Friedrich Stolz.
Actions in the body
Epinephrine plays a central role in the short-term stress reaction—the physiological response to threatening, exciting, or environmental stressor conditions such as high noise levels or bright light (see Fight-or-flight response). It is secreted by the adrenal medulla. When released into the bloodstream, epinephrine binds to multiple receptors and has numerous effects throughout the body. It increases heart rate and stroke volume, dilates the pupils, and constricts arterioles in the skin and gut while dilating arterioles in leg muscles. It elevates the blood sugar level by increasing catalysis of glycogen to glucose in the liver, and at the same time begins the breakdown of lipids in fat cells. Like some other stress hormones, epinephrine has a suppressive effect on the immune system.
Epinephrine is used as a drug to treat cardiac arrest and other cardiac dysrhythmias resulting in diminished or absent cardiac output; its action is to increase peripheral resistance via alpha-stimulated vasoconstriction, so that blood is shunted to the body's core. This beneficial action comes with a significant negative consequence—increased cardiac irritability—which may lead to additional complications immediately following an otherwise successful resuscitation. Alternatives to this treatment include vasopressin, a powerful antidiuretic which also increases peripheral vascular resistance leading to blood shunting via vasoconstriction, but without the attendant increase in myocardial irritability.
Because of its suppressive effect on the immune system, epinephrine is used to treat anaphylaxis and sepsis. Allergy patients undergoing immunotherapy may receive an epinephrine rinse before the allergen extract is administered, thus reducing the immune response to the administered allergen. It is also used as a bronchodilator for asthma if specific beta2-adrenergic receptor agonists are unavailable or ineffective. Adverse reactions to epinephrine include palpitations, tachycardia, anxiety, headache, tremor, hypertension, and acute pulmonary edema.
Biosynthesis
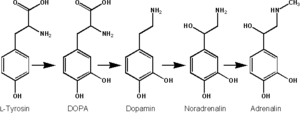
Epinephrine is synthesized from norepinephrine in a synthetic pathway shared by all catecholamines, including L-dopa, dopamine, norepinephrine, and epinephrine.
Epinephrine is synthesized via methylation of the primary distal amine of norepinephrine by phenylethanolamine N-methyltransferase (PNMT) in the cytosol of adrenergic neurons and cells of the adrenal medulla (so-called chromaffin cells). PNMT is only found in the cytosol of cells of adrenal medullary cells. PNMT uses S-adenosylmethionine (SAMe) as a cofactor to donate the methyl group to norepinephrine, creating epinephrine.
For norepinephrine to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H+ exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized epinephrine from the cytosol back into chromaffin granules in preparation for release.
Regulation
Epinephrine synthesis is solely under the control of the central nervous system (CNS). Several levels of regulation dominate epinephrine synthesis.
Adrenocorticotropic hormone (ACTH) and the sympathetic nervous system stimulate the synthesis of epinephrine precursors by enhancing the activity of enzymes involved in catecholamine synthesis. The specific enzymes are tyrosine hydroxylase in the synthesis of dopa and enzyme dopamine-β-hydroxylase in the synthesis of norepinephrine.
ACTH also stimulates the adrenal cortex to release cortisol, which increases the expression of PNMT in chromaffin cells, enhancing epinephrine synthesis.
The sympathetic nervous system, acting via splanchnic nerves to the adrenal medulla, stimulates the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of epinephrine (and norepinephrine) into the bloodstream.
Unlike many other hormones, epinephrine (and catecholamines in general) does not exert any negative feedback to down-regulate their own synthesis
A pheochromocytoma is a tumor of the adrenal gland (or, rarely, the ganglia of the sympathetic nervous system), which results in the uncontrolled secretion of catecholamines, usually epinephrine.
In liver cells, epinephrine binds to the β-Adrenergic receptor which changes conformation and helps Gs, a G protein, exchange GDP to GTP. This trimeric G protein dissociates to Gs alpha and Gs beta/gamma subunits. Ga alpha binds to adenyl cyclase thus converting ATP into Cyclic AMP. Cyclic AMP binds to the regulatory subunit of Protein Kinase A. Protein kinase A phosphorylate Phosphorylase Kinase. Meanwhile, Gs beta/gamma binds to the calcium channel channel and allows calcium ions to enter the cytoplasm. Calcium ions bind to calmodulin proteins, a protein present in all eukaryotic cells, which then binds tp Phosphorylase Kinase and finishes its activation. Phosphorylase Kinase phosphorylates Phosphorylase which then phosphorylates glycogen and converts it to glucose-6-phosphate.
Pharmacology
Epinephrine's actions are mediated through adrenergic receptors:
- It binds to α1 receptors of liver cells, which activate inositol-phospholipid signaling pathway, signaling the phosphorylation of insulin, leading to reduced ability of insulin to bind to its receptors.
- Epinephrine also activates β-adrenergic receptors of the liver and muscle cells, thereby activating the adenylate cyclase signaling pathway, which will in turn increase glycogenolysis.
β2 receptors are found primarily in skeletal muscle blood vessels where they trigger vasodilation. However, α-adrenergic receptors are found in most smooth muscles and splanchnic vessels, and epinephrine triggers vasoconstriction in those vessels.
Thus, depending on the patient, administration of epinephrine may raise or lower blood pressure, depending whether or not the net increase or decrease in peripheral resistance can balance the positive inotropic and chronotropic effects of epinephrine on the heart, effects which respectively increase the contractility and rate of the heart.
Terminology
Although widely referred to as adrenaline outside of the US, and the lay public worldwide, the USAN and INN for this chemical is epinephrine because adrenaline bore too much similarity to the Parke, Davis & Co trademark adrenalin (without the "e") which was registered in the US. The BAN and EP term for this chemical is adrenaline, and is indeed now one of the few differences between the INN and BAN systems of names.
Amongst US health professionals, the term epinephrine is used over adrenaline. However, it should be noted that universally, pharmaceuticals that mimic the effects of epinephrine are called adrenergics, and receptors for epinephrine are called adrenoceptors.
Isomers
Natural epinephrine is the R-(−)-L-epinephrine stereoisomer, see chirality.
Autoinjectors
Epinephrine is now also used in EpiPens and Twinjects. EpiPens are long narrow auto-injectors that administer epinephrine, Twinjects are similar but contain two doses of epinephrine.
Though both EpiPen and Twinject are trademark names, common usage of the terms are drifting toward the generic context of any epinephrine autoinjector.
See also
- EpiPen
- Twinject
- Anaphylaxis
- Adrenaline junkie
- Catechol-O-methyl transferase
- Noise health effects
ReferencesISBN links support NWE through referral fees
- ↑ Aronson JK (2000). "Where name and image meet" - the argument for "adrenaline". British Medical Journal 320, 506-9.
- ↑ Yamashima T (2003). Jokichi Takamine (1854-1922), the samurai chemist, and his work on adrenalin. J Med Biogr 11 (2): 95-102.
- ↑ Bennett M (1999). One hundred years of adrenaline: the discovery of autoreceptors. Clin Auton Res 9 (3): 145-59.
- Walter F. Boron, Emile L. Boulpaep (2005). Medical Physiology: A Cellular And Molecular Approach. Philadelphia, PA: Elsevier/Saunders. ISBN 1-4160-2328-3.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.