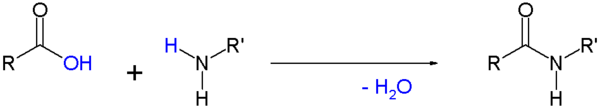Difference between revisions of "Amide" - New World Encyclopedia
({{Paid}}) |
m (Protected "Amide": Copyedited [edit=sysop:move=sysop]) |
||
| Line 1: | Line 1: | ||
| − | {{Claimed}}{{Contracted}}{{Images OK}}{{Submitted}}{{Approved}}{{Paid}} | + | {{Claimed}}{{Contracted}}{{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{Copyedited}} |
[[Image:Amide-general.png|right|thumb|right|200px|Amide functional group]] | [[Image:Amide-general.png|right|thumb|right|200px|Amide functional group]] | ||
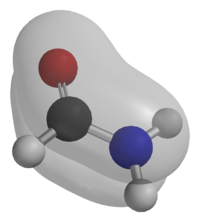
| − | [[Image:Formamide-MO-3D-balls.png|thumb|right|200px|Amides possess a [[conjugated system]] spread over the O, C and N atoms, consisting of [[molecular orbital]]s occupied by [[delocalized electron]]s. | + | [[Image:Formamide-MO-3D-balls.png|thumb|right|200px|Amides possess a [[conjugated system]] spread over the O, C and N atoms, consisting of [[molecular orbital]]s occupied by [[delocalized electron]]s. One of the [[pi bond|''π'' molecular orbitals]] in [[formamide]] is shown above.]] |
In [[chemistry]], an '''''amide''''' is one of two kinds of [[compound]]s: | In [[chemistry]], an '''''amide''''' is one of two kinds of [[compound]]s: | ||
| Line 8: | Line 8: | ||
Amides are the most stable of all the carbonyl functional groups. | Amides are the most stable of all the carbonyl functional groups. | ||
| − | Many chemists make a pronunciation distinction between the two, saying ({{IPA2|ə'mɪd}} for the [[carbonyl]]-[[nitrogen]] compound and ['æmɑɪd] for the [[anion]]. | + | Many chemists make a pronunciation distinction between the two, saying ({{IPA2|ə'mɪd}} for the [[carbonyl]]-[[nitrogen]] compound and ['æmɑɪd] for the [[anion]]. Others substitute one of these pronunciations with ['æmɪd], while still others pronounce both as ['æmɪd], making them [[homonym]]s. |
| − | In the first sense referred to above, an amide is an [[amine]] where one of the [[nitrogen]] [[substituent]]s is an [[acyl]] group; it is generally represented by the formula: R<sub>1</sub>([[carbon|C]][[oxygen|O]])NR<sub>2</sub>R<sub>3</sub> , where either or both R<sub>2</sub> and R<sub>3</sub> may be [[hydrogen]]. Specifically, an amide can also be regarded as a derivative of a [[carboxylic acid]] in which the [[hydroxyl]] group has been replaced by an amine or [[ammonia]].<br /> | + | In the first sense referred to above, an amide is an [[amine]] where one of the [[nitrogen]] [[substituent]]s is an [[acyl]] group; it is generally represented by the formula: R<sub>1</sub>([[carbon|C]][[oxygen|O]])NR<sub>2</sub>R<sub>3</sub>, where either or both R<sub>2</sub> and R<sub>3</sub> may be [[hydrogen]]. Specifically, an amide can also be regarded as a derivative of a [[carboxylic acid]] in which the [[hydroxyl]] group has been replaced by an amine or [[ammonia]].<br /> |
Compounds in which a [[hydrogen]] [[atom]] on [[nitrogen]] from [[ammonia]] or an [[amine]] is replaced by a [[metal]] [[cation]] are also known as amides or '''azanides'''. | Compounds in which a [[hydrogen]] [[atom]] on [[nitrogen]] from [[ammonia]] or an [[amine]] is replaced by a [[metal]] [[cation]] are also known as amides or '''azanides'''. | ||
| − | The second sense of the word ''amide'' is the amide [[anion]], which is a deprotonated form of [[ammonia]] (NH<sub>3</sub>) or an [[amine]]. | + | The second sense of the word ''amide'' is the amide [[anion]], which is a deprotonated form of [[ammonia]] (NH<sub>3</sub>) or an [[amine]]. It is generally represented by the formula: [R<sub>1</sub>NR<sub>2</sub>]<sup>-</sup>, and is an extremely strong [[Base (chemistry)|base]], due to the extreme weakness of ammonia and its [[Analog (chemistry)|analogue]]s as [[Brønsted acid]]s. |
| − | The remainder of this article is about the [[carbonyl]]-[[nitrogen]] sense of ''amide'' | + | The remainder of this article is about the [[carbonyl]]-[[nitrogen]] sense of ''amide.'' For examples of the [[anionic]] amide, see the articles [[Sodium amide]] and [[Lithium diisopropylamide]]. |
==Amide synthesis== | ==Amide synthesis== | ||
| Line 25: | Line 25: | ||
* Amides can be prepared from aryl alkyl ketones, sulfur and morpholine in the [[Willgerodt-Kindler reaction]] | * Amides can be prepared from aryl alkyl ketones, sulfur and morpholine in the [[Willgerodt-Kindler reaction]] | ||
* Other amide-forming [[Chemical reaction|reaction]]s are the [[Passerini reaction]] and the [[Ugi reaction]] | * Other amide-forming [[Chemical reaction|reaction]]s are the [[Passerini reaction]] and the [[Ugi reaction]] | ||
| − | * In the '''Bodroux reaction''' an amide RNHCOR' is synthesized from a [[carboxylic acid]] R-COOH and the the adduct of a [[Grignard reagent]] with an [[aniline]] derivative ArNHR' <ref>Bodroux | + | * In the '''Bodroux reaction''' an amide RNHCOR' is synthesized from a [[carboxylic acid]] R-COOH and the the adduct of a [[Grignard reagent]] with an [[aniline]] derivative ArNHR' <ref>F. Bodroux. ''Bull. Soc. Chim.'' (France, 1905) 33: 831;</ref> <ref> "Bodroux reaction at the Institute of Chemistry," (Skopje, Macedonia) [http://www.pmf.ukim.edu.mk/PMF/Chemistry/reactions/bodroux1.htm Link]Retrieved November 8, 2007. </ref> |
== Amide reactions == | == Amide reactions == | ||
| Line 33: | Line 33: | ||
* [[Hofmann rearrangement]] of primary amides to primary [[amine]]s. | * [[Hofmann rearrangement]] of primary amides to primary [[amine]]s. | ||
| − | Owing to their resonance stabilization, amides are relatively unreactive under physiological conditions, even less than similar compounds such as [[ester]]s. | + | Owing to their resonance stabilization, amides are relatively unreactive under physiological conditions, even less than similar compounds such as [[ester]]s. Nevertheless, amides can undergo chemical reactions, usually through an attack of an [[electronegativity|electronegative]] atom on the [[carbonyl]] [[carbon]], breaking the carbonyl double bond and forming a tetrahedral intermediate. When the functional group attacking the amide is a [[thiol]], [[hydroxyl]] or [[amine]], the resulting molecule may be called a [[cyclol]] or, more specifically, a thiacyclol, an oxacyclol or an azacyclol, respectively. |
| − | The proton of an amide does not dissociate readily under normal conditions; its p''K<sub>a</sub>'' is usually well above 15. | + | The proton of an amide does not dissociate readily under normal conditions; its p''K<sub>a</sub>'' is usually well above 15. However, under extremely acidic conditions, the carbonyl [[oxygen]] can become protonated with a p''K<sub>a</sub>'' of roughly -1. |
== Amide linkage (peptide bond) == | == Amide linkage (peptide bond) == | ||
| Line 41: | Line 41: | ||
== Amide properties == | == Amide properties == | ||
| − | [[Image:AmideResonance.png|right|400px|Amide resonance:]]Compared to [[amine]]s, amides are very weak [[Base (chemistry)|base]]s. While the [[conjugate acid]] of an amine has a [[pKa]] of about 9.5, the conjugate acid of an amide has a pKa around -0.5. Therefore amides don't have as clearly noticeable [[acid-base]] properties in [[water]]. This lack of basicity is explained by the [[electron]]-withdrawing nature of the [[carbonyl group]] where the lone pair of electrons on the [[nitrogen]] is delocalized by [[resonance (chemistry)|resonance]], thus forming a partial [[double bond]] with the [[carbonyl]] [[carbon]] and putting a negative charge on the [[oxygen]]. On the other hand, amides are much stronger bases than [[carboxylic acid]]s, [[ester]]s, [[aldehyde]]s, and [[ketone]]s (conjugated acid pKa between -6 and -10). It is estimated [[in silico]] that [[acetamide]] is represented by [[resonance structure]] A for 62 | + | [[Image:AmideResonance.png|right|400px|Amide resonance:]]Compared to [[amine]]s, amides are very weak [[Base (chemistry)|base]]s. While the [[conjugate acid]] of an amine has a [[pKa]] of about 9.5, the conjugate acid of an amide has a pKa around -0.5. Therefore amides don't have as clearly noticeable [[acid-base]] properties in [[water]]. This lack of basicity is explained by the [[electron]]-withdrawing nature of the [[carbonyl group]] where the lone pair of electrons on the [[nitrogen]] is delocalized by [[resonance (chemistry)|resonance]], thus forming a partial [[double bond]] with the [[carbonyl]] [[carbon]] and putting a negative charge on the [[oxygen]]. On the other hand, amides are much stronger bases than [[carboxylic acid]]s, [[ester]]s, [[aldehyde]]s, and [[ketone]]s (conjugated acid pKa between -6 and -10). It is estimated [[in silico]] that [[acetamide]] is represented by [[resonance structure]] A for 62 percent and by B for 28 percent <ref>Carl R. Kemnitz and Mark J. Loewen. "'Amide Resonance' Correlates with a Breadth of C-N Rotation Barriers." ''J. Am. Chem. Soc.'' 129(9)(2007): 2521-2528; (Article) {{DOI|10.1021/ja0663024}}</ref>. Resonance is largely prevented in the very strained [[quinuclidone]]. |
== Solubility == | == Solubility == | ||
| Line 47: | Line 47: | ||
While hydrogen bonding may enhance the water solubility of amides relative to hydrocarbons (alkanes, alkenes, alkynes and aromatic compounds), amides typically are regarded as compounds with low water solubility. They are significantly less water soluble than comparable acids or alcohols due to: | While hydrogen bonding may enhance the water solubility of amides relative to hydrocarbons (alkanes, alkenes, alkynes and aromatic compounds), amides typically are regarded as compounds with low water solubility. They are significantly less water soluble than comparable acids or alcohols due to: | ||
| − | 1) | + | 1.) their non-ionic character |
| − | 2) | + | 2.) the presence of nonpolar hydrocarbon functionality, and |
| − | 3) | + | 3.) the inability of tertiary amides to donate hydrogen bonds to water (they can only be H-bond acceptors). |
| − | Thus amides have water solubilities roughly comparable to esters. Typically amides are less soluble than comparable amines and carboxylic acids since these compounds can both donate and accept hydrogen bonds, and can ionize at appropriate pHs to further enhance solubility | + | Thus amides have water solubilities roughly comparable to esters. Typically amides are less soluble than comparable amines and carboxylic acids since these compounds can both donate and accept hydrogen bonds, and can ionize at appropriate pHs to further enhance solubility. |
==Derivatives== | ==Derivatives== | ||
| Line 64: | Line 64: | ||
== Notes == | == Notes == | ||
| − | + | ||
| + | <references/> | ||
== References == | == References == | ||
| − | * McMurry, John. 2004. ''Organic Chemistry'' | + | * McMurry, John. 2004. ''Organic Chemistry,'' 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052. |
| − | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry'' | + | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry,'' 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0136436692. |
| − | * Solomons, T.W. Graham, and | + | * Solomons, T.W. Graham, and Craig B. Fryhle. 2004. ''Organic Chemistry,'' 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998. |
==External links == | ==External links == | ||
Revision as of 15:46, 8 November 2007
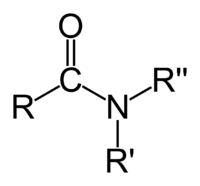
In chemistry, an amide is one of two kinds of compounds:
- the organic functional group characterized by a carbonyl group (C=O) linked to a nitrogen atom (N), or a compound that contains this functional group (pictured to the right); or
- a particular kind of nitrogen anion.
Amides are the most stable of all the carbonyl functional groups.
Many chemists make a pronunciation distinction between the two, saying (IPA: [ə'mɪd] for the carbonyl-nitrogen compound and ['æmɑɪd] for the anion. Others substitute one of these pronunciations with ['æmɪd], while still others pronounce both as ['æmɪd], making them homonyms.
In the first sense referred to above, an amide is an amine where one of the nitrogen substituents is an acyl group; it is generally represented by the formula: R1(CO)NR2R3, where either or both R2 and R3 may be hydrogen. Specifically, an amide can also be regarded as a derivative of a carboxylic acid in which the hydroxyl group has been replaced by an amine or ammonia.
Compounds in which a hydrogen atom on nitrogen from ammonia or an amine is replaced by a metal cation are also known as amides or azanides.
The second sense of the word amide is the amide anion, which is a deprotonated form of ammonia (NH3) or an amine. It is generally represented by the formula: [R1NR2]-, and is an extremely strong base, due to the extreme weakness of ammonia and its analogues as Brønsted acids.
The remainder of this article is about the carbonyl-nitrogen sense of amide. For examples of the anionic amide, see the articles Sodium amide and Lithium diisopropylamide.
Amide synthesis
- Amides are commonly formed from the reaction of a carboxylic acid with an amine. This is the reaction that forms peptide bonds between amino acids. These amides can participate in hydrogen bonding as hydrogen bond acceptors and donors, but do not ionize in aqueous solution, whereas their parent acids and amines are almost completely ionized in solution at neutral pH. Amide formation plays a role in the synthesis of some condensation polymers, such as nylon and Aramid (Twaron / Kevlar). In biochemistry peptides are synthesized in solid phase peptide synthesis.
- Cyclic amides are synthesized in the Beckmann rearrangement from oximes.
- Amides also form ketones in the Schmidt reaction
- Amides can be prepared from aryl alkyl ketones, sulfur and morpholine in the Willgerodt-Kindler reaction
- Other amide-forming reactions are the Passerini reaction and the Ugi reaction
- In the Bodroux reaction an amide RNHCOR' is synthesized from a carboxylic acid R-COOH and the the adduct of a Grignard reagent with an aniline derivative ArNHR' [1] [2]
Amide reactions
- Amide breakdown is possible via amide hydrolysis.
- In the Vilsmeier-Haack reaction an amide is converted into an imine.
- Hofmann rearrangement of primary amides to primary amines.
Owing to their resonance stabilization, amides are relatively unreactive under physiological conditions, even less than similar compounds such as esters. Nevertheless, amides can undergo chemical reactions, usually through an attack of an electronegative atom on the carbonyl carbon, breaking the carbonyl double bond and forming a tetrahedral intermediate. When the functional group attacking the amide is a thiol, hydroxyl or amine, the resulting molecule may be called a cyclol or, more specifically, a thiacyclol, an oxacyclol or an azacyclol, respectively.
The proton of an amide does not dissociate readily under normal conditions; its pKa is usually well above 15. However, under extremely acidic conditions, the carbonyl oxygen can become protonated with a pKa of roughly -1.
Amide linkage (peptide bond)
An amide linkage is kinetically stable to hydrolysis. However, it can be hydrolysed in boiling alkali, as well as in strong acidic conditions. Amide linkages in a biochemical context are called peptide linkages. Amide linkages constitute a defining molecular feature of proteins, the secondary structure of which is due in part to the hydrogen bonding abilities of amides.
Amide properties
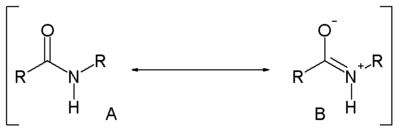
Compared to amines, amides are very weak bases. While the conjugate acid of an amine has a pKa of about 9.5, the conjugate acid of an amide has a pKa around -0.5. Therefore amides don't have as clearly noticeable acid-base properties in water. This lack of basicity is explained by the electron-withdrawing nature of the carbonyl group where the lone pair of electrons on the nitrogen is delocalized by resonance, thus forming a partial double bond with the carbonyl carbon and putting a negative charge on the oxygen. On the other hand, amides are much stronger bases than carboxylic acids, esters, aldehydes, and ketones (conjugated acid pKa between -6 and -10). It is estimated in silico that acetamide is represented by resonance structure A for 62 percent and by B for 28 percent [3]. Resonance is largely prevented in the very strained quinuclidone.
Solubility
Amides contain carbonyl (C=O) and ether (N-C) dipoles arising from covalent bonding between electronegative oxygen and nitrogen atoms and electro-neutral carbon atoms. Primary and secondary amides also contain two- and one N-H dipoles, respectively. Because of the pi-bonding arrangement of the carbonyl and the greater electronegativity of oxygen, the carbonyl (C=O) is a stronger dipole than the N-C dipole. The presence of a C=O dipole and, to a lesser extent a N-C dipole, allows amides to act as H-bond acceptors. In primary and secondary amides, the presence of N-H dipoles allows amides to function as H-bond donors as well. Thus amides can participate in hydrogen bonding with water and other protic solvents; the oxygen and nitrogen atoms can accept hydrogen bonds from water and the N-H hydrogen atoms can donate H-bonds. As a result of interactions such as these, the water solubility of amides is greater than that of corresponding hydrocarbons
While hydrogen bonding may enhance the water solubility of amides relative to hydrocarbons (alkanes, alkenes, alkynes and aromatic compounds), amides typically are regarded as compounds with low water solubility. They are significantly less water soluble than comparable acids or alcohols due to: 1.) their non-ionic character 2.) the presence of nonpolar hydrocarbon functionality, and 3.) the inability of tertiary amides to donate hydrogen bonds to water (they can only be H-bond acceptors). Thus amides have water solubilities roughly comparable to esters. Typically amides are less soluble than comparable amines and carboxylic acids since these compounds can both donate and accept hydrogen bonds, and can ionize at appropriate pHs to further enhance solubility.
Derivatives
Sulfonamides are analogues of amides in which the atom double-bonded to oxygen is sulfur rather than carbon.
Cyclic amides are called lactams.
Naming conventions
- Example: CH3CONH2 is named acetamide or ethanamide
- Other examples: propan-1-amide, N,N-dimethylpropanamide, acrylamide
- For more detail see IUPAC nomenclature of organic chemistry - Amines and Amides
Notes
- ↑ F. Bodroux. Bull. Soc. Chim. (France, 1905) 33: 831;
- ↑ "Bodroux reaction at the Institute of Chemistry," (Skopje, Macedonia) LinkRetrieved November 8, 2007.
- ↑ Carl R. Kemnitz and Mark J. Loewen. "'Amide Resonance' Correlates with a Breadth of C-N Rotation Barriers." J. Am. Chem. Soc. 129(9)(2007): 2521-2528; (Article) DOI:10.1021/ja0663024
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0136436692.
- Solomons, T.W. Graham, and Craig B. Fryhle. 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
External links
- IUPAC Compendium of Chemical Terminology - the Gold Book. International Union of Pure and Applied Chemistry. Retrieved June 4, 2007.
| Functional groups |
|---|
| Chemical class: Alcohol • Aldehyde • Alkane • Alkene • Alkyne • Amide • Amine • Azo compound • Benzene derivative • Carboxylic acid • Cyanate • Ester • Ether • Haloalkane • Imine • Isocyanide • Isocyanate • Ketone • Nitrile • Nitro compound • Nitroso compound • Peroxide • Phosphoric acid • Pyridine derivative • Sulfone • Sulfonic acid • Sulfoxide • Thioether • Thiol • Toluene derivative |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.