Testosterone
| |
Testosterone
| |
| Systematic name | |
| IUPAC name 17β-hydroxy-4-androsten-3-one | |
| Identifiers | |
| CAS number | 58-22-0 |
| ATC code | G03BA03 |
| PubChem | 6013 |
| Chemical data | |
| Formula | C19H28O2Â |
| Mol. weight | 288.43 |
| Physical data | |
| Melt. point | 155-156°C (-94°F) |
| Spec. rot | +110,2° |
| SEC Combust | â11080 kJ/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Liver, Testis and Prostate |
| Half life | 1-12 days |
| Excretion | Urine |
| Therapeutic considerations | |
| Pregnancy cat. | X (USA), Teratogenic effects |
| Legal status | Schedule III (USA) Schedule IV (Canada) |
| Routes | Intramuscular injection, transdermal (cream, gel, or patch), oral, sub-'Q' pellet |
Testosterone is a steroid hormone that acts in vertebrates to regulate many sexually dimorphic traits and express many fitness related traits in males (Zysline et al. 2006). A hormone is a secreted chemical messenger that coordinates cell-to-cell communication. Testosterone belongs to the class of steroid hormones known as androgensâthe generic term for any natural or synthetic compound that stimulates or controls the development and maintenance of masculine characteristics in vertebrates by binding to androgen receptors, including the activity of the accessory male sex organs and development of male secondary sex characteristics.
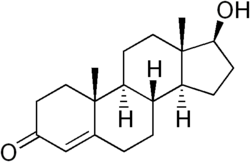
Steroid hormones such as testosterone (and estradiol on the female side) act early in development to organize male or female phenotypes that are activated later (Zysline et al. 2006). Testosterone's chemical formula is C19H28O2.
Although testosterone in vertebrates can activate male-typical phenotypes, testosterone's presence and action is not limited to males, and both sexes of most vertebrate taxa naturally produce testosterone (Zysline et al. 2006; Nelson 2000). In mammals, including humans, testosterone is primarily synthesized in the male's testes, but small amounts are also secreted by the female ovaries, the placenta, and the adrenal glands of both sexes.
Scientists have isolated testosterone and developed procedures for utilizing it medically to treat a wide variety of medical and psychological conditions, including low libido and even depression. This represents one aspect of human creativityâutilizing it in service to others. However, human creativity can also be applied toward ill purposes, and testosterone provides a good example. Despite known side effects, some have used testosterone and other steroids to gain unfair competitive advantage in sports. Some athletes have admitted winning competitions, such as track and field events while circumventing the rules with performance enhancing drugs, to the detriment of honest competitors. This case of sacrificing others and the sport for one's personal gain reveals an unethical application of human creativity.
Overview
Testosterone's effects can be classified as either anabolic (related to protein synthesis and growth) or virilizing (related to the biological development of male sex characteristics). However, the two categories are closely related:
- Anabolic effects involve growth of muscle mass, increased bone density, and stimulation of linear growth and bone maturation.
- Virilizing effects (also known as androgenic effects) include maturation of the sex organs, particularly the growth of the penis and the formation of the scrotum in the male fetus. During puberty, testosterone also coordinates development of masculine characteristics such as deepening of the voice and growth of facial hair.
Greatly differing amounts of testosterone prenatally, at puberty, and throughout life account for a share of biological differences between males and females. On average, the adult male human produces about 20 to 30 times the amount of testosterone synthesized by an adult female (Larsen, et al. 2002). Nonetheless, like men, women rely on testosterone (albeit in significantly smaller quantities) to maintain libido, bone density, and muscle mass throughout their lives.
Since testosterone was isolated by scientists in the 1930s, it has been used to treat a host of clinical issues, ranging from hypogonadism (the underproduction of natural testosterone) to certain forms of cancer, osteoporosis, and depression. More recently, testosterone replacement therapy has become available to older men, whose testosterone levels naturally decline with age; however, large-scale trials to assess the efficiency and long-term safety of this treatment are still lacking.
Anabolic steroids, a category which includes testosterone and its derivatives, have also received attention due to their controversial use to increase muscle mass and enhance athletic performance. Anabolic steroids were designated a controlled substance by the United States Congress in 1990, under the Anabolic Steroid Control Act; Canada, the United Kingdom, Australia, Argentina, and Brazil also have laws controlling their use and distribution (The Steroid Group, 2006).
Structure and classification
Like other steroid hormones, testosterone is derived from cholesterol, a sterol lipid with the chemical formula C27H45OH. Steroids are characterized by a carbon skeleton with four fused rings; they are distinguished by the functional groups attached to the rings.
Androgens (such as testosterone) are a major class of steroid hormones responsible for the development of male secondary sex characteristics. Testosterone is derived from the androgen androstenedione via a reduction of its 17-keto group.
The presence of a hydroxyl group (-OH) at position C-17 has enabled the development of synthetic forms of testosterone that can be administered in therapeutic treatments:
- Through the esterification (the substitution of an acid group for the hydroxyl group) of testosterone, the water solubility of the molecule is lowered and its lipid solubility increased, permitting a sterile oil-based injectable to form a âdepotâ in the muscle, from which it is gradually released.
- The hydroxyl side chain at the C-17 position also permits alkylation of the steroid molecule (substitution of an ethyl or methyl group for the hydroxyl group). Alkylation enables the development of oral steroids, which can be taken up by the digestive track, and hence are easily administered in pill form.
How testosterone works as a signaling molecule
Production and transport
Most hormones are synthesized in a specialized tissue, then released to target cells as needed. The largest amount of testosterone is produced by the testes in men, but it is also synthesized in smaller quantities in women by the thecal cells of the ovaries, the placenta, and the zona reticularis of the adrenal cortex in both sexes.
In the testes, testosterone is specifically produced by the Leydig cells. The male generative glands also contain the Sertoli cells, which require testosterone for spermatogenesis (the synthesis of spermatozoa).
Because testosterone is not soluble in water, it is transported to target cells bound to a specific plasma protein called sex hormone binding globulin (SHBG). When a hormone arrives at the target cell, it binds to, or âfits,â a site on the receptor protein. Binding creates a ligand-receptor complex, causing a conformational change (a change in the molecule's structural arrangement) that initiates a sequence of reactions leading to a change in cellular function.
Signaling
The effects of testosterone in humans and other vertebrates are triggered via two main mechanisms: (1) by activation of the androgen receptor and (2) by conversion of testosterone to the steroid estradiol, the major estrogen in humans, which in turn activates certain estrogen receptors.
- In the first method, free testosterone (T) is transported into the cytoplasm of target cells, where it can bind to the androgen receptor, or it can be reduced to 5Îą-dihydrotestosterone (DHT) by an enzyme in the cytoplasm. The resulting ligand-receptor complex undergoes a structural change that allows it to move into the nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA, affecting the expression of certain genes.
- In the second mechanism, which occurs primarily in bones and in the brain, testosterone is first converted to estradiol. In the bones, estradiol accelerates maturation of cartilage into bone, leading to closure of the epiphyses and conclusion of growth. In the central nervous system, estradiol rather than testosterone serves as the most important feedback signal to the hypothalamus. In many mammals, estradiol masculinizes sexually dimorphic areas of the brain in the male fetus.
Regulation
The rate of hormone biosynthesis and secretion is often regulated by feedback circuits, in which changes in the level of one hormone affects the levels of other hormones. Luteinizing hormone (LH), which is synthesized and secreted by the anterior lobe of the pituitary gland, functions in the regulation of testosterone levels. LH acts upon the Leydig cells of the testis to stimulate testosterone production. LH's release is controlled by pulses of gonadotropin-releasing hormone (GnRH) from the hypothalamus. These pulses, in turn, are subject to estrogen feedback from the gonads.
Role in human growth and development
Testosterone has its greatest impact on sexual differentiation during two stages of life: (1) Before birth and (2) during puberty.
Prenatal effects
Most prenatal androgen effects in humans occur between the 7th and 12th weeks of gestation, and are responsible for the masculinization of the developing fetus. Changes include closure of the perineum, thinning and rugation of the scrotum, growth of the penis, and closure of the urethral groove to the tip of the penis.
Prenatal virilization of genetic females and undervirilization of genetic males are common causes of ambiguous genitalia and intersex conditions. Undervirilization can occur if a genetic male cannot produce enough androgen or the body tissues are unable to respond to it. In females, intrauterine exposure to heightened levels of testosterone leads to profound genital abnormalities. In humans, for example, excessive exposure to androgens in the womb gives a girl a greatly enlarged clitoris and a vagina that is partially fused shut. In the most severe form of congenital adrenal hyperplasia, complete masculinization of a genetically female fetus results in an apparently normal baby boy with no palpable testes. More often, the virilization is partial and the genitalia are ambiguous.
Effects during puberty
Postnatal effects in both males and females are mostly dependent on the levels and duration of circulating free testosterone.
Early postnatal effects are the first visible effects of rising androgen levels in childhood, and occur in both boys and girls during puberty. They include adult-type body odor, increased oiliness of skin (acne), appearance of pubic hair and underarm hair, growth spurts (accelerated bone maturation), and the appearance of fine upper lip and sideburn hair.
In males, the following advanced postnatal effects typically manifest themselves during late puberty:
- Phallic enlargement
- Increased libido and frequency of erection
- Pubic hair extends to thighs and up toward umbilicus
- Facial hair (sideburns, beard, mustache)
- Chest hair, periareolar hair, perianal hair
- Subcutaneous fat in face decreases
- Increased muscle strength and mass
- Deepening of the voice
- Growth of the adam's apple
- Growth of spermatogenic tissue in testes; male fertility
- Growth of jaw, brow, chin, nose, and remodeling of facial bone contours
- Shoulders widen and rib cage expands
- Completion of bone maturation and termination of growth. This process occurs indirectly via estradiol metabolites, and hence, it tapers off more gradually in men than in women.
Adult testosterone effects are more clearly demonstrable in males than in females, but are likely important to both sexes. Some of these effects may decline as testosterone levels decrease in the later decades of adult life. They include maintenance of muscle mass, maintenance of bone density, libido, and clitoral engorgement/penile erection frequency.
Possible link to aggressive behavior
Behavioral effects of hormones are difficult to understand and to attribute to a given cause; in addition, a given hormone can have very different effects on behavior depending on the region of the central nervous system on which it acts. Nonetheless, high levels of circulating testosterone have been correlated to aggression in a variety of vertebrate species. For elephant bulls in musth (period condition in bull elephants), the amount of testosterone in the blood soars to levels fifty times higher than usual; during this period, male elephants demonstrate a mix of desperate lust and rage and are likely to engage in fights with other similarly affected males. Moreover, abnormal intrauterine exposure to androgens fosters aggression in females: Female mice that are snuggled between their brothers during fetal life are exposed to higher levels of androgens than females nestled between sisters and are more aggressive adults (Judson 2002).
The aggression associated with high levels of naturally circulating testosterone seems to be closely related to reproduction. For example, in male red-winged blackbirds (agelaius phoeniceus), testosterone levels peak during the two-week period when males are defending breeding territories and guarding their mates from rivals (Barnard 2003).
A experimental study on female dark-eyed juncos, a species of bird, found that exogenously increased testosterone levels led to increased intrasexual aggression (as well as decreased cell-mediated immune function) (Zysling et al. 2006). Increased aggressive behavior in female birds might help them acquire high quality mates or nesting sites, be more active in nest defense, or help win territorial interactions with other females, but might also lead to increased energy expenditure, potential for injury, or risk of predation (Zysling et al. 2006).
The use of synthetic testosterone and other anabolic steroids
Various forms of exogenous (externally produced) testosterone and other anabolic steroids are used in medical treatment and (more controversially) as a bodybuilding tool or performance enhancer; they are most commonly administered in oral, injectable, and transdermal form.
Therapeutic uses
Testosterone was originally used for the treatment of males who have little or no natural testosterone productionâthat is, males with hypogonadism. Hormone replacement therapy maintains blood testosterone levels in the normal range.
Over the years, testosterone has been administered to treat a variety of conditions, including infertility, lack of libido or erectile dysfunction, osteoporosis, and for penile enlargement, height growth, bone marrow stimulation and reversal of anemia, and even appetite stimulation.
To take advantage of its virilizing effects, testosterone is often administered to female-to-male transsexual men as part of hormone replacement therapy, with a "target level" of the normal male testosterone level.
Decline of testosterone production with age has led to a demand for androgen replacement therapy, though there is disagreement within the medical community about the efficacy and safety of such treatments. Caution about embracing testosterone replacement therapy stems in part from the lessons of female hormone replacement therapy trials, where initially promising results were later refuted by larger studies. Still, testosterone replacement therapies in women to treat or prevent osteoporosis have yet to show the risks now associated with estrogen replacement therapies.
Women may use testosterone to treat low libido, often a symptom or outcome of hormonal contraceptive use. Women may also use testosterone therapies to treat or prevent loss of bone density and muscle mass and to treat certain kinds of depression.
Some drugs work to reduce testosterone's effects. For example, finasteride inhibits the conversion of testosterone into its metabolite dihydrotestosterone (DHT). By lowering levels of DHT, finasteride may be used to treat benign prostatic hyperplasia (BPH) and androgenetic alopecia (male-pattern baldness).
Use in athletics and bodybuilding
Testosterone administered to an athlete in order to improve performance is considered to be a form of doping in most sports. After a series of scandals and publicity in the 1980s (such as runner Ben Johnson's improved performance at the 1988 Summer Olympics), prohibitions of anabolic steroid use were renewed or strengthened by many sports organizations. Testosterone and other anabolic steroids were designated a controlled substance by the United States Congress in 1990.
Side effects and risks of anabolic steroid use
Anabolic steroids have been associated with numerous side effects when administered in excessive doses; these include elevated cholesterol levels, acne, elevated blood pressure, hepatotoxicity, and alterations in left ventricle morphology. Adolescents who abuse anabolic steroids also risk stunted growth.
Some side effects are gender specific. Development of breast tissue in males, a condition called gynecomastia, is usually caused by high levels of circulating estrogen, the result of the increased conversion of testosterone to estrogen via an aromatase enzyme. Another male-specific side effect is testicular atrophy, a temporary reduction in the size of the testes. Possible female-specific side effects include increases in hair, deepening of the voice, enlarged clitoris (clitoral hypertrophy), as well as temporary decreases in menstrual cycles. When taken during pregnancy, anabolic steroids can affect fetal development.
A popular conception, perhaps misconception, regarding the side effects of anabolic steroids is that use leads to increased aggression, known in popular parlance as âroid rage.â Some early studies have shown a slight correlation between manic symptoms and anabolic steroid use; however, more comprehensive and recent studies have brought into question their methodology and conclusions (Pope and Katz 1988). Many scientists and medical professionals have concluded that anabolic steroids do not markedly increase aggressive behaviors (Fudala et al. 2003; Pope et al. 2000; OâConner 2002).
ReferencesISBN links support NWE through referral fees
- Barnard, C. 2004. Animal Behaviour: Mechanism, Development, Function and Evolution. Harlow, England: Pearson/Prentice Hall. ISBN 0130899364
- Fudala, P., R. Weinrieb, J. Calarco, K. Kampman, and C. Boardman. 2003. An evaluation of anabolic-androgenic steroid abusers over a period of 1 year: Seven case studies. Annals of Clinical Psychiatry 15(2): 121-30.
- Judson, O. 2002. Dr. Tatianaâs Sex Advice to All Creation: The Definitive Guide to the Evolutionary Biology of Sex. New York: Metropolitan Books. ISBN 0805063315
- Larsen, P. R., H. M. Kronenberg, S. Melmed, K. S. Polonsky, D. W. Foster, and J.D. Wilson. 2002. Williams Textbook of Endocrinology. London: Saunders. ISBN 0721692680
- Mutzebaugh, C. 1998. Does the choice of alpha-AAS really make a difference? HIV Hotline 8(5-6): 10-11.
- O'Connor, D., J. Archer, W. Hair, and F. Wu. 2002. Exogenous testosterone, aggression, and mood in eugonadal and hypogonadal men. Physiol. Behav 75(4): 557-566.
- Pope, H. and D. Katz. 1998. Affective and psychotic symptoms associated with anabolic steroid use. The American Journal of Psychiatry 145(4): 487-490.
- Pope, H. G., E. M. Kouri, and J. I. Hudson. 2000. Effects of Supraphysiologic Doses of Testosterone on Mood and Aggression in Normal Men. Med Sci Sports Exerc 57(2): 133-140.
- Steroids Working Group. 2006. 2006 Steroids report. United States Sentencing Commission. Retrieved June 22, 2007.
- Stryer, L. 1995. Biochemistry. New York: W.H. Freeman. ISBN 0716720094
- Zysling, D. A., T. J. Greives, C. W. Breuner, J. M. Casto, G. E. Demas, and E. D. Ketterson. 2006. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis). Hormones and Behavior 50: 200-207. Retrieved June 23, 2007.
External links
All links retrieved April 30, 2023.
| Hormones and endocrine glands - edit |
|---|
|
Hypothalamus: GnRH - TRH - CRH - GHRH - somatostatin - dopamine | Posterior pituitary: vasopressin - oxytocin | Anterior pituitary: GH - ACTH - TSH - LH - FSH - prolactin - MSH - endorphins - lipotropin Thyroid: T3 and T4 - calcitonin | Parathyroid: PTH | Adrenal medulla: epinephrine - norepinephrine | Adrenal cortex: aldosterone - cortisol - DHEA | Pancreas: glucagon- insulin - somatostatin | Ovary: estradiol - progesterone - inhibin - activin | Testis: testosterone - AMH - inhibin | Pineal gland: melatonin | Kidney: renin - EPO - calcitriol - prostaglandin | Heart atrium: ANP Stomach: gastrin | Duodenum: CCK - GIP - secretin - motilin - VIP | Ileum: enteroglucagon | Liver: IGF-1 Placenta: hCG - HPL - estrogen - progesterone Adipose tissue: leptin, adiponectin Target-derived NGF, BDNF, NT-3 |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Testosterone history
- Androgen history
- Anabolic_steroid history
- Virilization history
- Luteinizing_hormone history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.