Hypothalamus
| Brain: Hypothalamus | ||
|---|---|---|
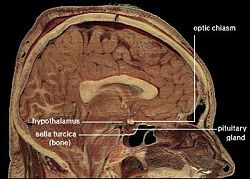
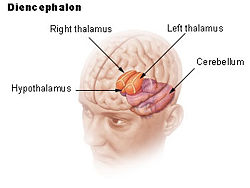
| Location of the human hypothalamus | ||
| Dienchephalon | ||
| Latin | hypothalamus | |
| Gray's | subject #189 812 | |
| NeuroNames | hier-358 | |
| MeSH | Hypothalamus | |
The hypothalamus, also known as the "master gland," is a supervising center in the brain that links the body's two control systems, the nervous system and the endocrine system, via interaction with the pituitary gland (hypophysis). The hypothalamus (from Greek áœÏÎżÎžÎ±Î»Î±ÎŒÎżÏ, "under the thalamus") is located below the thalamus, just above the brain stem, and occupies the major portion of the ventral region of the brain known as the diencephalon. The hypothalamus is found in all mammalian brains; in humans, it is roughly the size of an almond.
The hypothalamus gland regulates certain metabolic processes and other autonomic activities; it is a control center for functions of the autonomic nervous system. As needed, the hypothalamus synthesizes and secretes neurohormones, often called "releasing hormones," that control the secretion of hormones from the anterior pituitary gland.
The hypothalamus controls body temperature, hunger, thirst, blood pressure, heartbeat, carbohydrate and fat metabolism, and circadian cycles. Also, among other hormones, it releases gonadotropin releasing hormone (GnRH). The neurons that secrete GnRH are linked to the limbic system, which is primarily involved in the control of emotions and sexual activity. Specific functions are related to particular sections of the hypothalamus called nuclei.
Although the hypothalamus is envisioned as a "master gland," regulating such aspects as emotions (fear, rage) and sexual behavior, the typical religious conception of human beings is more complicated. Rather than seeing human beings as just a physical entity, governed by physical impulses, most religions depict each person as having a spiritual essence as well as a physical essence. Emotions and sexual activity are understood to be the result of an interaction of the physical (body) component of a human being (in this case, the hypothalamus) and the spiritual component (mind). While damage to the brain will interfere with this relationship, in healthy individuals actions take place based on this reciprocal, give and receive relationship between the spiritual and the physical.
Inputs
The hypothalamus is a very complex region, and even small nuclei within the hypothalamus are involved in many different functions. The paraventricular nucleus, for instance, contains oxytocin and vasopressin neurons which project to the posterior pituitary, but also contains neurons that regulate adrenocorticotropic hormone (ACTH) and thyroid-stimulating hormone (TSH) secretion (which project to the anterior pituitary), gastric reflexes, maternal behavior, blood pressure, food and liquid uptake, immune responses, and temperature.
The hypothalamus coordinates many seasonal and circadian rhythms, complex patterns of neuroendocrine outputs, complex homeostatic mechanisms, and many important stereotyped behaviors. The hypothalamus must therefore respond to many different signals, some of which are generated externally and some internally.
The hypothalamus thus is connected extensively with many parts of the central nervous system, including the brainstem reticular formation and autonomic zones, the limbic forebrain (particularly the amygdala, septum, diagonal band of Broca, and the olfactory bulbs), and the cerebral cortex.
The hypothalamus is responsive to:
- Light: Day length and photoperiod for generating circadian and seasonal rhythms
- Olfactory stimuli, including pheromones
- Steroids, including gonadal steroids and corticosteroids
- Neurally transmitted information arising in particular from the heart, the stomach, and the reproductive tract
- Autonomic inputs
- Blood-borne stimuli, including leptin, ghrelin, angiotensin, insulin, pituitary hormones, cytokines, plasma concentrations of glucose, and osmolarity etc.
- Stress
- Invading microorganisms by increasing body temperature, moving the body's thermostat upward.
Olfactory stimuli
Olfactory stimuli are important for reproduction and neuroendocrine function in many species. For instance, if a pregnant mouse is exposed to the urine of a "strange" male during a critical period after coitus, then the pregnancy fails (the Bruce effect). Thus during coitus, a female mouse forms a precise "olfactory memory" of her partner, which persists for several days.
Pheromonal cues aid synchronization of estrus in many species; in women, synchronized menstruation may also arise from pheromonal cues, although the role of pheromones in humans is contended by some.
Blood-borne stimuli
Peptide hormones have important influences upon the hypothalamus, and to do so they must evade the blood-brain barrier. The hypothalamus is bounded in part by specialized brain regions that lack an effective blood-brain barrier; the capillary endothelium at these sites is fenestrated to allow free passage of even large proteins and other molecules.
Some of these sites are the sites of neurosecretion: The neurohypophysis and the median eminence. However, others are sites at which the brain samples the composition of the blood. Two of these sites, the subfornical organ and the OVLT (organum vasculosum of the lamina terminalis) are so-called circumventricular organs, where neurons are in intimate contact with both blood and cerebrospinal fluid (CSF). These structures are densely vascularized, and contain osmoreceptive and sodium-receptive neurons that regulate fluid uptake (drinking), vasopressin release, sodium excretion, and sodium appetite. They also contain neurons with receptors for angiotensin, atrial natriuretic factor, endothelin, and relaxin, each of which is important in the regulation of fluid and electrolyte balance. Neurons in the OVLT and SFO project to the supraoptic nucleus and paraventricular nucleus, and also to preoptic hypothalamic areas. The circumventricular organs may also be the site of action of interleukins to elicit both fever and ACTH secretion, via effects on paraventricular neurons.
It is not clear how all peptides that influence hypothalamic activity gain the necessary access. In the case of prolactin and leptin, there is evidence of active uptake at the choroid plexus from blood into CSF. Some pituitary hormones have a negative feedback influence upon hypothalamic secretion; for example, growth hormone feeds back on the hypothalamus, but how it enters the brain is not clear. There is also evidence for central actions of prolactin and thyroid-stimulating hormone (TSH).
Steroids
The hypothalamus contains neurons that are sensitive to gonadal steroids and glucocorticoids (the steroid hormones of the adrenal gland, released in response to ACTH). It also contains specialized glucose-sensitive neurons (in the arcuate nucleus and ventromedial hypothalamus), which are important for appetite. The preoptic area contains thermosensitive neurons; these are important for thyrotropin-releasing hormone (TRH) secretion.
Neural inputs
The hypothalamus receives many inputs from the brainstem; notably from the nucleus of the solitary tract, the locus coeruleus, and the ventrolateral medulla. Oxytocin secretion in response to suckling or vagino-cervical stimulation is mediated by some of these pathways; vasopressin secretion in response to cardiovascular stimuli arising from chemoreceptors in the carotid sinus and aortic arch, and from low-pressure atrial volume receptors, is mediated by others. In the rat, stimulation of the vagina also causes prolactin secretion, and this results in pseudo-pregnancy following an infertile mating. In the rabbit, coitus elicits reflex ovulation. In the sheep, cervical stimulation in the presence of high levels of estrogen can induce maternal behavior in a virgin ewe. These effects are all mediated by the hypothalamus, and the information is carried mainly by spinal pathways that relay in the brainstem. Stimulation of the nipples stimulates release of oxytocin and prolactin and suppresses the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Cardiovascular stimuli are carried by the vagus nerve, but the vagus also conveys a variety of visceral information, including, for instance, signals arising from gastric distension to suppress feeding. Again this information reaches the hypothalamus via relays in the brainstem.
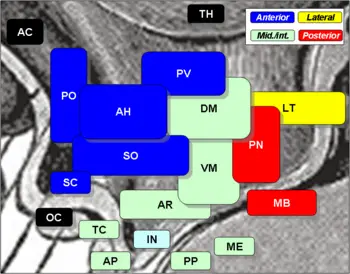
Nuclei
The hypothalamic nuclei include the following:
| Region | Medial Area | Lateral Area |
| Anterior |
Medial preoptic nucleus |
Lateral preoptic nucleus |
| Tuberal |
Dorsomedial nucleus |
Lateral nucleus |
| Posterior |
Mammillary nuclei (part of mammillary bodies) |
Lateral nucleus |
Outputs
The outputs of the hypothalamus can be divided into two categories: Neural projections and endocrine hormones (Weedman Molavi 1997).
Projections
Most fiber systems of the hypothalamus run in two ways (bidirectional).
- Projections to areas caudal to the hypothalamus go through the medial forebrain bundle, the mammillotegmental tract, and the dorsal longitudinal fasciculus.
- Projections to areas rostral to the hypothalamus are carried by the mammillothalamic tract, the fornix, and stria terminalis.
Hormones
Most of the hormones generated in the hypothalamus are distributed to the pituitary via the hypophyseal portal system (Bowen 1998).
The primary hypothalamic hormones are:
| Hormone | Location |
|---|---|
| Somatostatin | periventricular nucleus |
| Growth hormone-releasing hormone | arcuate nucleus |
| Gonadotropin-releasing hormone | medial preoptic nucleus |
| Gonadotropin-releasing hormone | arcuate nucleus |
| Thyrotropin-releasing hormone | paraventricular |
| Thyrotropin-releasing hormone | anterior hypothalamic nuclei |
| Corticotropin-releasing hormone | paraventricular nucleus |
| Dopamine | arcuate nucleus |
| Melatonin | suprachiasmatic nucleus |
| Oxytocin | supraoptic nucleus |
| Vasopressin | supraoptic nucleus |
Control of food intake
The extreme lateral part of the ventromedial nucleus of the hypothalamus is responsible for the control of food intake. Stimulation of this area causes increased food intake, while bilateral lesion of this area causes complete cessation of food intake. Medial parts of the nucleus have a controlling effect on the lateral part. Bilateral lesion of the medial part of the ventromedial nucleus causes hyperphagia and obesity of the animal. Further lesion of the lateral part of the ventromedial nucleus in the same animal produces complete cessation of food intake.
There are different hypotheses related to this regulation (Theologides 1976):
- Lipostatic hypothesis. This hypothesis holds that adipose tissue produces a humoral signal that is proportionate to the amount of fat and acts on the hypothalamus to decrease food intake and increase energy output. It has been evident that a hormone leptin acts on the hypothalamus to decrease food intake and increase energy output.
- Gutpeptide hypothesis. Gastrointestinal hormones like Grp, glucagons, CCK, and others are claimed to inhibit food intake. The food entering the gastrointestinal tract triggers the release of these hormones, which acts on the brain to produce satiety. The brain contains both CCK-A and CCK-B receptors.
- Glucostatic hypothesis. The activity of the satiety center in the ventromedial nuclei is probably governed by the glucose utilization in the neurons. It has been postulated that when their glucose utilization is low and consequently when the arteriovenous blood glucose difference across them is low, the activity across the neurons decrease. Under these conditions, the activity of the feeding center is unchecked and the individual feels hungry. Food intake is rapidly increased by intraventricular administration of 2-deoxyglucose, therefore decreasing glucose utilization in cells.
- Thermostatic hypothesis. According to this hypothesis, a decrease in body temperature below a given set point stimulates appetite, while an increase above the set point inhibits appetite.
Sexual dimorphism
Several hypothalamic nuclei are sexually dimorphic. In other words, there are clear differences in both structure and function between males and females.
Some differences are apparent even in gross neuroanatomy: Most notable is the sexually dimorphic nucleus within the preoptic area, which is present only in males. However, most of the differences are subtle changes in the connectivity and chemical sensitivity of particular sets of neurons.
The importance of these changes can be recognized by functional differences between males and females. For instance, the pattern of secretion of growth hormone is sexually dimorphic, and this is one reason why in many species, adult males are much larger than females.
Responses to ovarian steroids
A striking functional dimorphism is in the behavioral responses to ovarian steroids of the adult. Males and females respond differently to ovarian steroids, partly because the expression of estrogen-sensitive neurons in the hypothalamus is sexually dimorphic. In other words, estrogen receptors are expressed in different sets of neurons.
Estrogen and progesterone act by influencing gene expression in particular neurons. To influence gene expression, estrogen binds to an intracellular receptor, and this complex is translocated to the cell nucleus where it interacts with regions of the DNA known as estrogen regulatory elements (EREs). Increased protein synthesis may follow as soon as 30 min later.
Thus, for estrogen to influence the expression of a particular gene in a particular cell, the following must occur:
- The cell must be exposed to estrogen
- The cell must express estrogen receptors
- The gene must be one that is regulated by an ERE.
Male and female brains differ in the distribution of estrogen receptors, and this difference is an irreversible consequence of neonatal steroid exposure. Estrogen receptors (and progesterone receptors) are found mainly in neurons in the anterior and mediobasal hypothalamus, notably:
- the preoptic area (where Gonadotropin-releasing hormone 1 (LHRH) neurons are located)
- the periventricular nucleus (where somatostatin neurons are located)
- the ventromedial hypothalamus (which is important for sexual behavior).
Gonadal steroids in neonatal life of rats
In neonatal life, gonadal steroids influence the development of the neuroendocrine hypothalamus. For instance, they determine the ability of females to exhibit a normal reproductive cycle, and of males and females to display appropriate reproductive behaviors in adult life.
- If a female rat is injected once with testosterone in the first few days of postnatal life (during the "critical period" of sex-steroid influence), the hypothalamus is irreversibly masculinized; the adult rat will be incapable of generating an LH surge in response to estrogen (a characteristic of females), but will be capable of exhibiting male sexual behaviors (mounting a sexually receptive female).
- By contrast, a male rat castrated just after birth will be feminized, and the adult will show female sexual behavior in response to estrogen (sexual receptivity, lordosis}.
Androgens in primates
In primates, the developmental influence of androgens is less clear, and the consequences are less complete. "Tomboyism" in girls might reflect the effects of androgens on the fetal brain, but the sex of rearing during the first 2-3 years is believed by many to be the most important determinant of gender identity, because during this phase either estrogen or testosterone will have permanent effects on either a female or male brain, influencing both heterosexuality and homosexuality.
The paradox is that the masculinizing effects of testosterone are mediated by estrogen. Within the brain, testosterone is aromatized to estradiol, which is the principal active hormone for developmental influences. The human testis secretes high levels of testosterone from about week 8 of fetal life until 5-6 months after birth (a similar perinatal surge in testosterone is observed in many species), a process that appears to underlie the male phenotype. Estrogen from the maternal circulation is relatively ineffective, partly because of the high circulating levels of steroid-binding proteins in pregnancy.
Other influences upon hypothalamic development
Sex steroids are not the only important influences upon hypothalamic development; stress (positive or negative) in early life determines the capacity of the adult hypothalamus to respond to an acute stressor. Unlike gonadal steroid receptors, glucocorticoid receptors are very widespread throughout the brain; in the paraventricular nucleus, they mediate negative feedback control of corticotropin-releasing hormone (CRF) synthesis and secretion, but elsewhere their role is not well understood.
Effects of aging on the Hypothalamus
Studies in female mice have shown that both Supraoptic nucleus (SON) and Paraventricular nucleus (PVN) lose approximately one-third of IGF-1R immunoreactive cells with normal aging. Also, Old caloricly restricted (CR) mice lost higher numbers of IGF-1R non-immunoreactive cells while maintaining similar counts of IGF-1R immunoreactive cells in comparison to Old-Al mice. Consequently, Old-CR mice show a higher percentage of IGF-1R immunoreactive cells reflecting increased hypothalamic sensitivity to IGF-1 in comparison to normally aging mice (Saeed et al. 2007; Yaghmaie et al. 2006; Yaghmaie et al. 2007).
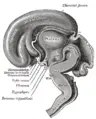
Additional images
ReferencesISBN links support NWE through referral fees
- Bowen, R. 1998. Overview of hypothalamic and pituitary hormone. Colorado State University. Retrieved May 19, 2007.
- Saeed, O., F. Yaghmaie, S. A. Garan, A. M. Gouw, M. A. Voelker, H. Sternberg, and P. S. Timiras. 2007. Insulin-like growth factor-1 receptor immunoreactive cells are selectively maintained in the paraventricular hypothalamus of calorically restricted mice. Int J Dev Neurosci 25(1): 23-28.
- Theologides, A. 1976. Anorexia-producing intermediary metabolites. Am J Clin Nutr 29(5): 552-558.
- Weedman Molavi, D. 1997. Hypothalamus and autonomic nervous system. Neuroscience tutorial, The Washington University School of Medicine.
- Yaghmaie, F., O. Saeed, S. A. Garan, M. A. Voelker, A. M. Gouw, W. Freitag, H. Sternberg, and P. S. Timiras. 2006. Age-dependent loss of insulin-like growth factor-1 receptor immunoreactive cells in the supraoptic hypothalamus is reduced in calorically restricted mice. Int J Dev Neurosci 24(7): 431-436.
- Yaghmaie, F., O. Saeed, S. A. Garan, A. M. Gouw, P. Jafar, J. Kaur, S. Nijjar, P. S. Timiras, H. Sternberg, and M. A. Voelker. 2007. Tracking changes in hypothalamic IGF-1 sensitivity with aging and caloric restriction. Experimental Gerontology 42(1-2): 148-149.
| Endocrine system - edit |
|---|
| Adrenal gland | Corpus luteum | Hypothalamus | Kidney | Ovaries | Pancreas | Parathyroid gland | Pineal gland | Pituitary gland | Testes | Thyroid gland |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.