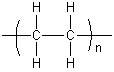
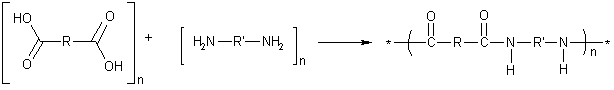Polymer
A polymer is a chemical compound consisting of large molecules, each of which is a long chain made up of small structural units that are linked together by covalent chemical bonds. Each structural unit, called a monomer, is a small molecule of low-to-moderate molecular weight. Within a given polymer molecule, the monomers are usually identical or similar in structure. The chemical reaction by which monomers are linked together to form polymers is called polymerization.
- Intro:Polymers form a large, diverse group of substances, ranging from flexible proteins to stiff, high-strength Kevlar fibers.
The term polymer is derived from the Greek words polys, meaning "many," and meros, meaning "parts" [1]. Likewise, the term monomer is derived from two Greek words, monos and meros, where monos means "alone" or single."
Monomers that are similar in structure usually have different chemical substituents attached to a particular basic structure. The differences between monomers can affect properties of the polymer, such as its solubility, flexibility, and strength. In the case of proteins, the monomers are called amino acids. The amino acids share a basic structure but have different chemical side groups known as side chains. The differences between amino acids give each protein molecule the ability to adopt a biologically active three-dimensional structure, called its conformation.
Identical monomers with nonreactive side chains produce a polymer chain that tends to adopt a random coil conformation, as described by an ideal chain mathematical model.
Although most polymers are organic, with carbon-based monomers, there are also inorganic polymers, such as the silicones, which have a backbone of alternating silicon and oxygen atoms.
Classification and nomenclature
Polymers are typically classified as follows:
- Thermoplastics: A thermoplastic is a material that is deformable, melts to a liquid when heated, and freezes to a brittle, glassy state when cooled sufficiently. Most thermoplastics are polymers whose molecules have linear or branched structures. The molecules associate with one another through various interactions: weak van der Waals forces, as in the case of polyethylene and polypropylene; stronger dipole-dipole interactions; hydrogen bonding, as in the case of nylon; or the stacking of aromatic rings, as in the case of polystyrene.
- Thermosets (or thermosetting plastics): These are materials that are taken through a "curing" process with the addition of energy. The energy may be in the form of heat (generally above 200 °C), a chemical reaction, or irradiation. Thermoset materials are usually liquidy, powdery, or malleable prior to curing, and designed to be molded into their final form or used as adhesives. During the curing process, molecules of the starting material become cross-linked and take on a stronger form. Once cured, the thermoset cannot be remelted and remolded. Examples of thermosets are vulcanized rubber, Bakelite™ (used in electrical insulators), melamine (used in worktop surfaces), and epoxy resin (used as an adhesive).
- Elastomers: The term elastomer is applied to an "elastic polymer"—that is, a polymer that returns to its original shape when a load is removed. Elastomers are usually thermosets (that require curing), but some are thermoplastic. The long polymer chains become cross-linked during curing and account for the flexible nature of the material. The molecular form of elastomers has been likened to a "spaghetti and meatball" structure, where the meatballs signify cross-links between the flexible spaghetti strands (polymer chains). Most elastomers are rubbers, and the term elastomer is often used interchangeably with the term rubber. Examples of thermoplastic elastomers are Hytrel® and Santoprene®.
- Coordination polymers: In a coordination polymer, many metal centers are interconnected through ligand bridges. Most of the common halides and oxides are coordination polymers. In a more conventional sense, the term coordination polymer is reserved for compounds where the metals are bridged by polyatomic ligands, such as cyanide and carboxylates. One of the most popular bridging ligands used in the synthesis of these polymers is a tricarboxylic acid called BTC (benzene-1,3,5-tricarboxylic acid). The polymers are metal salts of this acid. Another coordination polymer is Prussian Blue, which is based on Fe-CN-Fe linkages.
Polymers are often named in terms of the monomer from which they are made. For example, polyethene (also called polyethylene) is the name given to the polymer formed when thousands of ethene (ethylene) molecules are bonded together. The polyethene molecules are straight or branched chains of repeating -CH2-CH2- units (with a -CH3 at each terminal). The polymerization reaction can be written as follows.
The product may also be written as:
Proteins are polymers of amino acids. Typically, hundreds of the (nominally) twenty different amino acid monomers make up a protein chain, and the sequence of monomers determines its shape and biological function. (There are also shorter oligopeptides which function as hormones.) But there are active regions, surrounded by, as is believed now (Aug 2003), structural regions, whose sole role is to expose the active regions. (There may be more than one on a given protein.) So the exact sequence of amino acids in certain parts of the chains can vary from species to species, and even given mutations within a species, so long as the active sites are properly accessible.
- Whereas the formation of polyethylene occurs spontaneously under the right conditions, the synthesis of biopolymers such as proteins and nucleic acids requires the help of enzyme catalysts, substances that facilitate and accelerate reactions. Unlike synthetic polymers, these biopolymers have exact sequences and lengths. (This does not include the carbohydrates.) Since the 1950s, catalysts have also revolutionised the development of synthetic polymers. By allowing more careful control over polymerization reactions, polymers with new properties, such as the ability to emit colored light, have been manufactured.
Copolymerization
Copolymerization involves the linking together of two or more different monomers, producing chains with varied properties. For example, a protein can be called a copolymer—one in which different amino acid monomers are linked together. Depending on the sequence of amino acids, the protein chains have different shapes and functions.
When ethene is copolymerized with small amounts of 1-hexene (or 4-methyl-1-pentene), the product is called linear low-density polyethene (LLDPE). The C4 branches resulting from the hexene lower the density and prevent large crystalline regions from forming in the polymer, as they do in high-density polyethene (HDPE). This means that LLDPE can withstand strong tearing forces while maintaining flexibility.
The polymerization reaction may be carried out in a stepwise manner, to produce a structure with long sequences (or blocks) of one monomer alternating with long sequences of the other. The product is called a block copolymer.
In the case of some copolymers, called graft copolymers, entire chains of one kind (such as polystyrene) are made to grow out of the sides of chains of another kind (such as polybutadiene). The resultant product is less brittle and more impact-resistant. Thus, block and graft copolymers can combine the useful properties of both constituents and often behave as quasi-two-phase systems.
The formation of nylon is an example of step-growth polymerization, or condensation polymerization. The two types of monomers can have different R and R' groups, shown in the diagram below. The properties of the nylon can vary, depending on the R and R' groups in the monomers used.
The first commercially successful, completely synthetic polymer was nylon 6,6, with four carbon atoms in the R group (adipic acid) and six carbon atoms in the R' group (hexamethylene diamine). Each monomer actually contributes 6 carbon atoms (including the two carboxyl carbons of adipic acid)—hence the name nylon 6,6. In naming nylons, the number of carbons from the diamine is given first, and the number from the diacid, second. Kevlar is an aromatic nylon in which both R and R' are benzene rings.
Copolymers illustrate the point that the repeating unit in a polymer—such as a nylon, polyester, or polyurethane—is often made up of two (or more) monomers.
Physical properties of polymers
Physical properties of polymers include
- Degree of polymerization: This is the number of monomer units in an average polymer chain, at time t in a polymerization reaction. For most industrial purposes, synthetic polymer chains need to have thousands or tens of thousands of monomer units.
- Molar mass distribution: Polymer chains in a solution or substance are generally not of equal length. By contrast, each smaller molecules in which every atom is stoichiometrically accounted for, and each molecule has a set molecular mass. An ensemble of differing chain lengths, often obeying a normal (Gaussian) distribution, occurs because polymer chains terminate during polymerization after random amounts of chain lengthening (propagation).
- crystallinity, as well as the thermal phase transitions:
- Tg, glass transition temperature
- Tm, melting point (for thermoplastics).
- Branching During the propagation of polymer chains, branching can occur. In free-radical polymerization, this occurs when a chain curls back and bonds to an earlier part of the chain. When this curl breaks, it leaves small chains sprouting from the main carbon backbone. Branched carbon chains cannot line up as close to each other as unbranched chains can. This causes less contact between atoms of different chains, and fewer opportunities for induced or permanent dipoles to occur. A low density results from the chains being further apart. Lower melting points and tensile strengths are evident, because the intermolecular bonds are weaker and require less energy to break. Besides branching, polymers can have other topologies: linear, network (cross-linked 3D structure), IPN (integrated polymer network), comb, or star as well as dendrimer and hyperbranched structures.
- Stereoregularity or tacticity describes the isomeric arrangement of functional groups on the backbone of carbon chains.
Chemical properties of polymers
The attractive forces between polymer chains play a large part in determining a polymer's properties. Given that polymer chains are so long, these interchain forces are amplified far beyond the attractions between conventional molecules. Also, longer chains are more amorphous (randomly oriented). Polymers can be visualized as tangled spaghetti chains—the more tangled the chains, the more difficult it is to pull any one strand out. These stronger forces typically result in high tensile strength and melting points.
The intermolecular forces in polymers are determined by dipoles in the monomer units. For example, polymers containing amide groups can form hydrogen bonds between adjacent chains. The somewhat positively charged hydrogen atoms in the N-H groups of one chain are strongly attracted to the somewhat negatively charged oxygen atoms in the C=O groups on another. Such strong hydrogen bonds are responsible for the high tensile strength and melting point of Kevlar.
In the case of polyesters, there is dipole-dipole bonding between the oxygen atoms in C=O groups and the hydrogen atoms in C-H groups. Dipole bonding is not as strong as hydrogen bonding, so the polyester's melting point and strength are lower than Kevlar's, but polyesters have greater flexibility.
If one considers polyethene, the monomer units (ethene) have no permanent dipole. Attractive forces between polyethene chains arise from weak van der Waals forces. Molecules can be thought of as being surrounded by a cloud of negative electrons. As two polymer chains approach, their electron clouds repel one another. This has the effect of lowering the electron density on one side of a polymer chain, creating a slight positive charge on this side. This charge is enough to attract the second polymer chain. Van der Waals forces are quite weak, however, so polyethene melts at low temperatures.
See also
- Biopolymer
- Electroactive polymers
- Polymer chemistry
- Polymerization
- Polymer physics
- Important publications in polymer chemistry
External links
- Polymer dictionary
- Responsive Biopolymers for Drug Delivery and Imaging
- Chemical Resistance of Fluoropolymers
- Polymer Chemistry Hypertext, Educational resource
- Polymer Chemistry Innovations
- Materials for Organic devices
- The Macrogalleria - a cyberwonderland of polymer fun!
- Polymer & Plastics Glossary
- International Journal of Polymer Analysis and Characterization
- International Journal of Polymeric Materials
- Polymer-Plastics Technology and Engineering
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.