Difference between revisions of "Butane" - New World Encyclopedia
(importing article, credit) |
(ready) |
||
| Line 1: | Line 1: | ||
| + | {{Ready}} | ||
{{Chembox new | {{Chembox new | ||
| Name = Butane | | Name = Butane | ||
| Line 56: | Line 57: | ||
==Effects and health issues== | ==Effects and health issues== | ||
| − | Inhaling butane can cause [[drowsiness]], [[narcosis]], [[asphyxia]]; [[cardiac arrhythmia]] and [[frostbite]], which can result in instant death from [[Asphyxiation]], [[Acute toxicity]] and [[ventricular fibrillation]]. Butane is the most commonly misused volatile solvent in the UK, and was the cause of 52% of solvent related deaths in 2000.<ref> [http://www.sgul.ac.uk/dms/AF54AFD9D207A9A41D353717989DC4E0.pdf Trends in death Associated with Abuse of Volatile Substances 1971-2004] Field-Smith M, Bland JM, Taylor JC, et al., Department of Public Health Sciences. London: St George’s Medical School</ref> By spraying butane directly into the throat, the jet of fluid can cool rapidly to –20 °C by expansion, causing prolonged [[laryngospasm]].<ref name="multiple">Ramsey J, Anderson HR, Bloor K, et al. An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse. Hum Toxicol 1989;8:261–9</ref> "[[Sudden Sniffer's Death]] syndrome" | + | Inhaling butane can cause [[drowsiness]], [[narcosis]], [[asphyxia]]; [[cardiac arrhythmia]] and [[frostbite]], which can result in instant death from [[Asphyxiation]], [[Acute toxicity]] and [[ventricular fibrillation]]. Butane is the most commonly misused volatile solvent in the UK, and was the cause of 52% of solvent related deaths in 2000.<ref>[http://www.sgul.ac.uk/dms/AF54AFD9D207A9A41D353717989DC4E0.pdf Trends in death Associated with Abuse of Volatile Substances 1971-2004] Field-Smith M, Bland JM, Taylor JC, et al., Department of Public Health Sciences. London: St George’s Medical School Retrieved November 8, 2007.</ref> By spraying butane directly into the throat, the jet of fluid can cool rapidly to –20 °C by expansion, causing prolonged [[laryngospasm]].<ref name="multiple">Ramsey J, Anderson HR, Bloor K, et al. An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse. Hum Toxicol 1989;8:261–9</ref> "[[Sudden Sniffer's Death]] syndrome," first described by Bass in 1970,<ref>Bass M. Sudden sniffing death. JAMA 1970;212:2075–9</ref> is the most common single cause of solvent related death, resulting in 55% of known fatal cases.<ref name="multiple"/> |
[[Image:ButaneGasCylinder.jpg|thumb|right|Butane gas cylinder used for cooking]] | [[Image:ButaneGasCylinder.jpg|thumb|right|Butane gas cylinder used for cooking]] | ||
| Line 64: | Line 65: | ||
* [[Camping Gaz]] | * [[Camping Gaz]] | ||
* [[Calor]] gas | * [[Calor]] gas | ||
| − | |||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
| Line 73: | Line 70: | ||
==External links== | ==External links== | ||
| + | All links retrieved November 8, 2007. | ||
*[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc02/icsc0232.htm International Chemical Safety Card 0232] | *[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc02/icsc0232.htm International Chemical Safety Card 0232] | ||
*[http://www.cdc.gov/niosh/npg/npgd0068.html NIOSH Pocket Guide to Chemical Hazards] | *[http://www.cdc.gov/niosh/npg/npgd0068.html NIOSH Pocket Guide to Chemical Hazards] | ||
| Line 79: | Line 77: | ||
*[http://www.bluerhinos.co.uk/molview/indv.php?id=11 Molview from bluerhinos.co.uk] See Butane in 3D | *[http://www.bluerhinos.co.uk/molview/indv.php?id=11 Molview from bluerhinos.co.uk] See Butane in 3D | ||
* [http://www.compchemwiki.org/index.php?title=Butane Computational Chemistry Wiki] | * [http://www.compchemwiki.org/index.php?title=Butane Computational Chemistry Wiki] | ||
| − | |||
*[http://www.worldlpgas.com World LP Gas Association (WLPGA)] | *[http://www.worldlpgas.com World LP Gas Association (WLPGA)] | ||
*[http://www.lpga.co.uk/LPGA.htm LP Gas Association: Propane and Butane in the UK] | *[http://www.lpga.co.uk/LPGA.htm LP Gas Association: Propane and Butane in the UK] | ||
| Line 89: | Line 86: | ||
{{E number infobox 930-949}} | {{E number infobox 930-949}} | ||
| − | [[Category: | + | [[Category:Physical sciences]] |
| − | [[Category: | + | [[Category:Chemistry]] |
| − | |||
{{Credit|169189716}} | {{Credit|169189716}} | ||
Revision as of 00:58, 8 November 2007
| Butane | |
|---|---|
| |
| |
| Identifiers | |
| CAS number | [] |
| SMILES | CCCC |
| Properties | |
| Molecular formula | C4H10 |
| Molar mass | 58.14 g/mol |
| Appearance | Colorless gas |
| Density | 2.48 g/l, gas (15 °C, 1 atm) |
| Melting point |
−138 °C (135 K) |
| Boiling point |
−0.5 °C (272.6 K) |
| Solubility in water | 6.1 mg/100 ml (20 °C) |
| Hazards | |
| EU classification | Highly flammable (F+) |
| NFPA 704 |
|
| Flash point | −60 °C |
| Related Compounds | |
| Related alkanes | Propane; Pentane |
| Related compounds | Isobutane |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
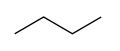
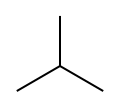
Butane, also called n-butane, is the unbranched alkane with four carbon atoms, CH3CH2CH2CH3. Butane is also used as a collective term for n-butane together with its only other isomer, isobutane (also called methylpropane), CH(CH3)3.
Butanes are highly flammable, colorless, easily liquefied gases. The name butane was derived by back-formation from the name of butyric acid.
- Structures of the two isomers of butane
Reactions and uses
When oxygen is plentiful, butane burns to form carbon dioxide and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide may also be formed.
- 2C4H10 + 13O2 → 8CO2 + 10H2O
n-Butane is the feedstock for DuPont's catalytic process for the preparation of maleic anhydride:
- CH3CH2CH2CH3 + 3.5O2 → C2H2(CO)2O + 4H2O
n-Butane, like all hydrocarbons, undergoes free radical chlorination providing both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative rates of the chlorination is partially explained by the differing bond dissociation energies, 425 and 411 kJ/mol for the two types of C-H bonds. The two central carbon atoms have the slightly weaker C-H bonds.
Butane gas is sold bottled as a fuel for cooking and camping. When blended with Propane and other hydrocarbons, it is referred to commercially as LPG. It is also used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for cigarette lighters and as a propellant in aerosol sprays.
Very pure forms of butane, especially isobutane, can be used as refrigerants and have largely replaced the ozone layer depleting halomethanes, for instance in household refrigerators and freezers. The flammability of butane is not a major issue because the amount of butane in an appliance is not enough to cause a combustible mix given the amount of air in a room. The system operating pressure for butane is lower than for the halomethanes, such as R-12, so direct conversion of R-12 systems to butane, such as in automotive air conditioning systems, will not function optimally.
Effects and health issues
Inhaling butane can cause drowsiness, narcosis, asphyxia; cardiac arrhythmia and frostbite, which can result in instant death from Asphyxiation, Acute toxicity and ventricular fibrillation. Butane is the most commonly misused volatile solvent in the UK, and was the cause of 52% of solvent related deaths in 2000.[1] By spraying butane directly into the throat, the jet of fluid can cool rapidly to –20 °C by expansion, causing prolonged laryngospasm.[2] "Sudden Sniffer's Death syndrome," first described by Bass in 1970,[3] is the most common single cause of solvent related death, resulting in 55% of known fatal cases.[2]
See also
- Volatile substance abuse
- Camping Gaz
- Calor gas
ReferencesISBN links support NWE through referral fees
- ↑ Trends in death Associated with Abuse of Volatile Substances 1971-2004 Field-Smith M, Bland JM, Taylor JC, et al., Department of Public Health Sciences. London: St George’s Medical School Retrieved November 8, 2007.
- ↑ 2.0 2.1 Ramsey J, Anderson HR, Bloor K, et al. An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse. Hum Toxicol 1989;8:261–9
- ↑ Bass M. Sudden sniffing death. JAMA 1970;212:2075–9
External links
All links retrieved November 8, 2007.
- International Chemical Safety Card 0232
- NIOSH Pocket Guide to Chemical Hazards
- European Chemicals Bureau
- n-Butane Molecule of the Month
- Molview from bluerhinos.co.uk See Butane in 3D
- Computational Chemistry Wiki
- World LP Gas Association (WLPGA)
- LP Gas Association: Propane and Butane in the UK
- Global BioSciences In-Situ Bioremediation utilizing Butane
- Butane Viscosity as tunction of temperature and pressure
| Alkanes | |||||||||||||||||||||||||||||||
|
methane |
| |
ethane |
| |
propane |
| |
butane |
| |
pentane |
| |
hexane |
|||||||||||||||||||||
|
heptane |
| |
octane |
| |
nonane |
| |
decane |
| |
undecane |
| |
dodecane |
| ||||||||||||||||||||
E numbers
| ||
|---|---|---|
| Colours (E100-199) • Preservatives (E200-299) • Antioxidants & Acidity regulators (E300-399) • Thickeners, stabilisers & emulsifiers (E400-499) • pH regulators & anti-caking agents (E500-599) • Flavour enhancers (E600-699) • Miscellaneous (E900-999) • Additional chemicals (E1100-1599) | ||
| Waxes (E900-909) • Synthetic glazes (E910-919) • Improving agents (E920-929) • Packaging gases (E930-949) • Sweeteners (E950-969) • Foaming agents (E990-999) | ||
| Argon (E938) • Helium (E939) • Dichlorodifluoromethane (E940) • Nitrogen (E941) • Nitrous oxide (E942) • Butane (E943a) • Isobutane (E943b) • Propane (E944) • Oxygen (E948) • Hydrogen (E949) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.