Aging
Currently working on —Jennifer Tanabe May 2021.
Aging or ageing is the process of becoming older. The term refers especially to humans, many other animals, and fungi. In the broader sense, aging can refer to single cells within an organism which have ceased dividing (cellular senescence) or to the population of a species (population aging).
In humans, aging represents the accumulation of changes in a human being over time and can encompass physical, psychological, and social changes. Reaction time, for example, may slow with age, while memories and general knowledge typically increase.
Definitions
Mortality can be used to define biological aging, which refers to an organism's increased rate of death as it progresses throughout its lifecycle and increases its chronological age.[1] Another possible way to define aging is through functional definitions, of which there are two main types: The first describes how varying types of deteriorative changes that accumulate in the life of a post-maturation organism can leave it vulnerable, leading to a decreased ability of the organism to survive. The second is a senescence-based definition which describes age-related changes in an organism that increase its mortality rate over time by negatively affecting its vitality and functional performance.[1]
An important distinction to make is that biological aging is not the same thing as the accumulation of diseases related to old age; disease is a blanket term used to describe a process within an organism that causes a decrease in its functional ability.[1] Aging is the natural and inevitable biological process that all animal life, including human beings go through from conception through birth to death. While death by other external causes, such as disease, accident, predation, and so forth, is common, it would occur naturally due to aging if such causes were absent.
Biological basis
The causes of aging are uncertain;[2] current theories are assigned to the damage concept, whereby the accumulation of damage (such as DNA oxidation) may cause biological systems to fail, or to the programmed aging concept, whereby problems with the internal processes (epigenomic maintenance such as DNA methylation[3]) may cause aging. Programmed aging should not be confused with programmed cell death (apoptosis). Additionally, there can be other reasons, which can speed up the rate of aging in organisms including human beings like obesity[4][5] and compromised immune system.
Researchers are only just beginning to understand the biological basis of aging even in relatively simple and short-lived organisms, such as yeast.[6] Less still is known of mammalian aging, in part due to the much longer lives of even small mammals such as the mouse (around 3 years). A model organism for studying of aging is the nematode C. elegans. Thanks to its short lifespan of 2–3 weeks, our ability to easily perform genetic manipulations or to suppress gene activity with RNA interference, or other factors.[7] Most known mutations and RNA interference targets that extend lifespan were first discovered in C. elegans.[8]
The factors proposed to influence biological aging[9] fall into two main categories, programmed and damage-related. Programmed factors follow a biological timetable, perhaps one that might be a continuation of the one that regulates childhood growth and development. This regulation would depend on changes in gene expression that affect the systems responsible for maintenance, repair and defense responses. Damage-related factors include internal and environmental assaults to living organisms that induce cumulative damage at various levels.[10] A third, novel, concept is that aging is mediated by vicious cycles.[11]
In a detailed review, Lopez-Otin and colleagues (2013), who discuss ageing through the lens of the damage theory, propose nine metabolic "hallmarks" of ageing in various organisms but especially mammals:[12]
- genomic instability (mutations accumulated in nuclear DNA, in mtDNA, and in the nuclear lamina)
- telomere attrition (the authors note that artificial telomerase confers non-cancerous immortality to otherwise mortal cells)
- epigenetic alterations (including DNA methylation patterns, post-translational modification of histones, and chromatin remodelling)
- loss of proteostasis (protein folding and proteolysis)
- deregulated nutrient sensing (relating to the Growth hormone/Insulin-like growth factor 1 signalling pathway, which is the most conserved ageing-controlling pathway in evolution and among its targets are the FOXO3/Sirtuin transcription factors and the mTOR complexes, probably responsive to caloric restriction)
- mitochondrial dysfunction (the authors point out however that a causal link between ageing and increased mitochondrial production of reactive oxygen species is no longer supported by recent research)
- cellular senescence (accumulation of no longer dividing cells in certain tissues, a process induced especially by p16INK4a/Rb and p19ARF/p53 to stop cancerous cells from proliferating)
- stem cell exhaustion (in the authors' view caused by damage factors such as those listed above)
- altered intercellular communication (encompassing especially inflammation but possibly also other intercellular interactions)
There are three main metabolic pathways which can influence the rate of ageing:
- the FOXO3/Sirtuin pathway, probably responsive to caloric restriction
- the Growth hormone/Insulin-like growth factor 1 signalling pathway
- the activity levels of the electron transport chain in mitochondria[13] and (in plants) in chloroplasts.
It is likely that most of these pathways affect ageing separately, because targeting them simultaneously leads to additive increases in lifespan.[14]
Programmed factors
The rate of ageing varies substantially across different species, and this, to a large extent, is genetically based. For example, numerous perennial plants ranging from strawberries and potatoes to willow trees typically produce clones of themselves by vegetative reproduction and are thus potentially immortal, while annual plants such as wheat and watermelons die each year and reproduce by sexual reproduction. In 2008 it was discovered that inactivation of only two genes in the annual plant Arabidopsis thaliana leads to its conversion into a potentially immortal perennial plant.[15] The oldest animals known so far are 15,000-year-old Antarctic sponges,[16] which can reproduce both sexually and clonally.
Clonal immortality apart, there are certain species whose individual lifespans stand out among Earth's life-forms, including the bristlecone pine at 5062 years[17] or 5067 years,[16] invertebrates like the hard clam (known as quahog in New England) at 508 years,[18] the Greenland shark at 400 years,[19] various deep-sea tube worms at over 300 years,[20] fish like the sturgeon and the rockfish, and the sea anemone[21] and lobster.[22][23] Such organisms are sometimes said to exhibit negligible senescence.[24] The genetic aspect has also been demonstrated in studies of human centenarians.
- Evolution of ageing: Many have argued that life span, like other phenotypes, is selected. Traits that benefit early survival and reproduction will be selected for even if they contribute to an earlier death. Such a genetic effect is called the antagonistic pleiotropy effect when referring to a gene (pleiotropy signifying the gene has a double function – enabling reproduction at a young age but costing the organism life expectancy in old age) and is called the disposable soma effect when referring to an entire genetic programme (the organism diverting limited resources from maintenance to reproduction).[25] The biological mechanisms which regulate lifespan evolved several hundred million years ago.[8]
- Some evidence is provided by oxygen-deprived bacterial cultures.[26]
- The theory would explain why the autosomal dominant disease, Huntington's disease, can persist even though it is inexorably lethal. Also, it has been suggested that some of the genetic variants that increase fertility in the young increase cancer risk in the old. Such variants occur in genes p53[27] and BRCA1.[28]
- The reproductive-cell cycle theory argues that ageing is regulated specifically by reproductive hormones that act in an antagonistic pleiotropic manner via cell cycle signalling, promoting growth and development early in life to achieve reproduction, but becoming dysregulated later in life, driving senescence (dyosis) in a futile attempt to maintain reproductive ability.[29][30] The endocrine dyscrasia that follows the loss of follicles with menopause, and the loss of Leydig and Sertoli cells during andropause, drive aberrant cell cycle signalling that leads to cell death and dysfunction, tissue dysfunction (disease) and ultimately death. Moreover, the hormones that regulate reproduction also regulate cellular metabolism, explaining the increases in fat deposition during pregnancy through to the deposition of centralised adiposity with the dysregulation of the HPG axis following menopause and during andropause (Atwood and Bowen, 2004). This theory, which introduced a new definition of ageing, has facilitated the conceptualisation of why and how ageing occurs at the evolutionary, physiological and molecular levels.[29]
- Autoimmunity: The idea that ageing results from an increase in autoantibodies that attack the body's tissues. A number of diseases associated with ageing, such as atrophic gastritis and Hashimoto's thyroiditis, are probably autoimmune in this way. However, while inflammation is very much evident in old mammals, even completely immunodeficient mice raised in pathogen-free laboratory conditions still experience senescence.[citation needed]
- The cellular balance between energy generation and consumption (energy homeostasis) requires tight regulation during ageing. In 2011, it was demonstrated that acetylation levels of AMP-activated protein kinase change with age in yeast and that preventing this change slows yeast ageing.[31]
- Skin ageing is caused in part by TGF-β, which reduces the subcutaneous fat that gives skin a pleasant appearance and texture. TGF-β does this by blocking the conversion of dermal fibroblasts into fat cells; with fewer fat cells underneath to provide support, the skin becomes saggy and wrinkled. Subcutaneous fat also produces cathelicidin, which is a peptide that fights bacterial infections.[32][33]
- DNA damage theory of ageing: DNA damage is thought to be the common basis of both cancer and ageing, and it has been argued that intrinsic causes of DNA damage are the most important drivers of ageing.[34][35][36] Genetic damage (aberrant structural alterations of the DNA), mutations (changes in the DNA sequence), and epimutations (methylation of gene promoter regions or alterations of the DNA scaffolding which regulate gene expression), can cause abnormal gene expression. DNA damage causes the cells to stop dividing or induces apoptosis, often affecting stem cell pools and hence hindering regeneration. However, lifelong studies of mice suggest that most mutations happen during embryonic and childhood development, when cells divide often, as each cell division is a chance for errors in DNA replication.[37]
- Genetic instability: Dogs annually lose approximately 3.3% of the DNA in their heart muscle cells while humans lose approximately 0.6% of their heart muscle DNA each year. These numbers are close to the ratio of the maximum longevities of the two species (120 years vs. 20 years, a 6/1 ratio). The comparative percentage is also similar between the dog and human for yearly DNA loss in the brain and lymphocytes. As stated by lead author, Bernard L. Strehler, "... genetic damage (particularly gene loss) is almost certainly (or probably the) central cause of ageing."[38]
- Accumulation of waste:
- A buildup of waste products in cells presumably interferes with metabolism. For example, a waste product called lipofuscin is formed by a complex reaction in cells that binds fat to proteins. This waste accumulates in the cells as small granules, which increase in size as a person ages.[39]
- The hallmark of ageing yeast cells appears to be overproduction of certain proteins.[6]
- Autophagy induction can enhance clearance of toxic intracellular waste associated with neurodegenerative diseases and has been comprehensively demonstrated to improve lifespan in yeast, worms, flies, rodents and primates. The situation, however, has been complicated by the identification that autophagy up-regulation can also occur during ageing.[40] Autophagy is enhanced in obese mice by caloric restriction, exercise, and a low fat diet (but in these mice is evidently not related with the activation of AMP-activated protein kinase, see above).[41]
- Wear-and-tear theory: The very general idea that changes associated with ageing are the result of chance damage that accumulates over time.[10]
- Accumulation of errors: The idea that ageing results from chance events that escape proof reading mechanisms, which gradually damages the genetic code.
- Heterochromatin loss, model of ageing.[42][43][44]
- Transposable elements in genome disintegration as the primary role in the mechanism of ageing.[45][46][47]
- Cross-linkage: The idea that ageing results from accumulation of cross-linked compounds that interfere with normal cell function.[48][49]
- Studies of mtDNA mutator mice have shown that increased levels of somatic mtDNA mutations directly can cause a variety of ageing phenotypes. The authors propose that mtDNA mutations lead to respiratory-chain-deficient cells and thence to apoptosis and cell loss. They cast doubt experimentally however on the common assumption that mitochondrial mutations and dysfunction lead to increased generation of reactive oxygen species (ROS).[50]
- Free-radical theory: Damage by free radicals, or more generally reactive oxygen species or oxidative stress, create damage that may give rise to the symptoms we recognise as ageing.[48][51] Michael Ristow's group has provided evidence that the effect of calorie restriction may be due to increased formation of free radicals within the mitochondria, causing a secondary induction of increased antioxidant defence capacity.[52]
- Mitochondrial theory of ageing: free radicals produced by mitochondrial activity damage cellular components, leading to ageing.
- DNA oxidation and caloric restriction: Caloric restriction reduces 8-OH-dG DNA damage in organs of ageing rats and mice.[53][54] Thus, reduction of oxidative DNA damage is associated with a slower rate of ageing and increased lifespan.[55] In a 2021 review article, Vijg stated that “Based on an abundance of evidence, DNA damage is now considered as the single most important driver of the degenerative processes that collectively cause aging.”[56]
Effects

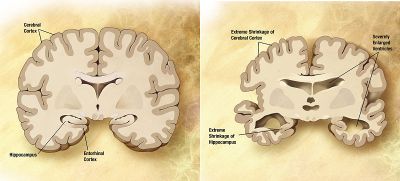
Aging increases the risk of diseases.
A number of characteristic aging symptoms are experienced by a significant proportion of human beings during their lifetimes, including the following:
- After peaking in the mid-20s, female fertility declines.[59]
- After age 30 the mass of human body is decreased until 70 years and then shows damping oscillations.[58]
- Muscles have reduced capacity of responding to exercise or injury and loss of muscle mass and strength (sarcopenia) is common.[60]
- Hand strength and mobility are decreased during the aging process. These things include "hand and finger strength and ability to control submaximal pinch force and maintain a steady precision pinch posture, manual speed, and hand sensation."[61]
- People over 35 years of age are at increasing risk for losing strength in the ciliary muscle of the eyes which leads to difficulty focusing on close objects, or presbyopia.[62] Most people experience presbyopia by age 45–50.
- Around age 50, hair turns grey. Pattern hair loss or baldness by the age of 50 affects about 50 percent of men and 25 percent of women.
- Menopause typically occurs between 44 and 58 years of age.
- In the 60–64 age cohort, the incidence of osteoarthritis rises.
- Wrinkles develop mainly due to photoageing, particularly affecting sun-exposed areas (face).
- Almost half of people older than 75 have hearing loss (presbycusis) inhibiting spoken communication.[63]
- By age 80, more than half of all Americans either have a cataract or have had cataract surgery.[64]
- Frailty, a syndrome of decreased strength, physical activity, physical performance and energy, affects 25 percent of those over 85.[65]
- Atherosclerosis is classified as an aging disease, which leads to cardiovascular disease (for example stroke and heart attack), globally the most common causes of death.[66]
- Dementia becomes more common with age. The spectrum ranges from mild cognitive impairment to the neurodegenerative diseases of Alzheimer's disease, cerebrovascular disease, Parkinson's disease and Lou Gehrig's disease. bout 3 percent of people between the ages of 65 and 74, 19 percent between 75 and 84, and nearly half of those over 85 years of age have dementia.[67] Furthermore, many types of memory decline with aging, but not semantic memory or general knowledge such as vocabulary definitions, which typically increases or remains steady until late adulthood.[68] Individual variations in rate of cognitive decline may be explained in terms of people having different lengths of life.[69]
Prevention and delay
Lifestyle
A healthy diet may reduce the effects of aging. For example, the Mediterranean diet is credited with lowering the risk of heart disease and early death. The major contributors to mortality risk reduction appear to be a higher consumption of vegetables, fish, fruits, nuts, and monounsaturated fatty acids (olive oil).[70]
Amount of sleep is related to mortality. People who live the longest report sleeping for six to seven hours each night, while the National Sleep Foundation recommends eight hours of sleep per night for optimal health. However, this range of sleep has not been shown to be causal in increasing life span, merely correlated with longer life which may be affected by various other factors. Studies linking longer or shorter sleep patterns to increased mortality do not necessarily imply that people should change their existing, and comfortable, sleep patterns. [71]
Physical exercise may increase life expectancy.[72] People who participate in moderate to high levels of physical exercise have a lower mortality rate compared to individuals who are not physically active.[73] Moderate levels of exercise have been correlated with preventing ageing and improving quality of life by reducing inflammatory potential.[74] The majority of the benefits from exercise are achieved with around 3500 metabolic equivalent (MET) minutes per week.[75] For example, climbing stairs 10 minutes, vacuuming 15 minutes, gardening 20 minutes, running 20 minutes, and walking or bicycling for 25 minutes on a daily basis would together achieve about 3000 MET minutes a week.[75] Other research seems to suggest a relationship between regular physical exercise and cognitive functioning in old age.[76]
Avoidance of chronic stress (as opposed to acute stress) is associated with a slower loss of telomeres in most but not all studies,[77][78] and with decreased cortisol levels. A chronically high cortisol level compromises the immune system, causes cardiac damage/arterosclerosis and is associated with facial ageing, and the latter in turn is a marker for increased morbidity and mortality.[79][80] A meta-analysis shows that loneliness carries a higher mortality risk than smoking.[81] Stress can be countered by social connection, spirituality, and (for men more clearly than for women) married life, all of which are associated with longevity.[82][83][84][85]
Medical intervention
Theoretically, extension of maximum lifespan in humans could be achieved by reducing the rate of aging damage by periodic replacement of damaged tissues, molecular repair or rejuvenation of deteriorated cells and tissues, reversal of harmful epigenetic changes, or the enhancement of enzyme telomerase activity. Research geared towards life extension strategies in various organisms is currently under way at a number of academic and private institutions. Various drugs and interventions have been shown to slow or reverse the biological effects of aging in animal models, but none has yet been proven to do so in humans.
Society and culture
Different cultures express age in different ways. Most legal systems define a specific age for when an individual is allowed or obliged to do particular activities. These age specifications include voting age, drinking age, age of consent, age of majority, age of criminal responsibility, marriageable age, age of candidacy, and mandatory retirement age. In other words, chronological aging may be distinguished from "social aging" (cultural age-expectations of how people should act as they grow older) and "biological aging" (an organism's physical state as it ages).[86]
Sociology
In the fielda of sociology and mental health, ageing is seen in five different views: aging as maturity, aging as decline, aging as a life-cycle event, aging as generation, and aging as survival.[87] Positive correlates with aging often include economics, employment, marriage, children, education, and sense of control. Retirement, a common transition faced by the elderly, may have both positive and negative consequences.[88]
Population aging is the increase in the number and proportion of older people in society. Population aging occurs through migration, longer life expectancy (decreased death rate), and decreased birth rate. As a population ages, it has a significant impact on society. Young people tend to have fewer legal privileges (if they are below the age of majority), they are more likely to push for political and social change, to develop and adopt new technologies, and to need education. Older people have different requirements from society and government, and frequently have differing values as well, such as for property and pension rights.[89]
Economics
Among the most urgent concerns of older persons worldwide is income security. This poses challenges for governments with aging populations to ensure that investment in pension systems is sufficient to provide economic independence and reduce poverty in old age. These challenges vary for developing and developed countries:
Sustainability of these systems is of particular concern, particularly in developed countries, while social protection and old-age pension coverage remain a challenge for developing countries, where a large proportion of the labour force is found in the informal sector.[90]
Health care demand
With age inevitable biological changes occur that increase the risk of illness and disability. A number of health problems become more prevalent as people get older. These include mental health problems as well as physical health problems, especially dementia.
While the effects on society of population aging are multidimensional, there is no doubt about its impact on health care demand:
A life-cycle approach to health care – one that starts early, continues through the reproductive years and lasts into old age – is essential for the physical and emotional well-being of older persons, and, indeed, all people. Public policies and programmes should additionally address the needs of older impoverished people who cannot afford health care.[90]
Self-perception
As humans age, their bodies begin to break down, and among other changes their skin begins to look different. However, especially in Western culture, these changes are not always appreciated and being young or at least youthful is celebrated especially when young people are successful.[91]
Generally, aversion to aging and its effects is primarily a Western attitude. In other places around the world, old age is celebrated and honored. In Korea, for example, a special party called hwangap is held to celebrate and congratulate an individual for turning 60 years old.[92]
Positive self-perceptions of aging are associated with better mental and physical health and well-being.[93] Positive self-perception of health has been correlated with higher well-being and reduced mortality among the elderly. Various reasons have been proposed for this association; people who are objectively healthy may naturally rate their health better as than that of their ill counterparts.
As people age, subjective health remains relatively stable, even though objective health worsens. In fact, perceived health improves with age when objective health is controlled in the equation. This phenomenon is known as the "paradox of aging," and may be a result of social comparison; for instance, the older people get, the more they may consider themselves in better health than their same-aged peers. Elderly people often associate their functional and physical decline with the normal aging process.[94]
Successful aging
Traditional definitions of successful aging have emphasized absence of physical and cognitive disabilities.[95] Successful aging has been characterized as involving three components: a) freedom from disease and disability, b) high cognitive and physical functioning, and c) social and productive engagement.[96]
Notes
- ↑ 1.0 1.1 1.2 Roger B. McDonald, Biology of Aging (Garland Science, 2019, ISBN 0815345674).
- ↑ Liochev, Stefan I. (2015-12-17). Which Is the Most Significant Cause of Aging?. Antioxidants 4 (4): 793–810.
- ↑ (2016)Epigenomic maintenance through dietary intervention can facilitate DNA repair process to slow down the progress of premature aging. IUBMB Life 68 (9): 717–721.
- ↑ (May 2019) 'Obesageing': Linking obesity & ageing. The Indian Journal of Medical Research 149 (5): 610–615.
- ↑ (2019-05-03) Obesity May Accelerate the Aging Process. Frontiers in Endocrinology 10.
- ↑ 6.0 6.1 (December 2015) Protein biogenesis machinery is a driver of replicative aging in yeast. eLife 4: e08527.
- ↑ (2012) "Analysis of Aging in Caenorhabditis elegans", Caenorhabditis Elegans: Cell Biology and Physiology. Academic Press, 353–81. ISBN 978-0-12-394620-1.
- ↑ 8.0 8.1 (October 2009) Extreme-longevity mutations orchestrate silencing of multiple signaling pathways. Biochimica et Biophysica Acta (BBA) - General Subjects 1790 (10): 1075–83.
- ↑ Mitochondrial Theory of Aging and Other Aging Theories. 1Vigor.
- ↑ 10.0 10.1 (October 2010) Modern Biological Theories of Aging. Aging and Disease 1 (2): 72–74.
- ↑ (January 2019) Age-related diseases as vicious cycles. Ageing Research Reviews 49: 11–26.
- ↑ (June 2013) The hallmarks of aging. Cell 153 (6): 1194–217.
- ↑ (2008). Molecular Biology of Aging. Cell 96 (2): 347–62.
- ↑ (May 2011) Aging as an event of proteostasis collapse. Cold Spring Harbor Perspectives in Biology 3 (5): a004440.
- ↑ (December 2008)Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nature Genetics 40 (12): 1489–92.
- ↑ 16.0 16.1 "The oldest living thing on Earth", BBC News, 12 June 2017.
- ↑ Oldlist. Rocky Mountain Tree Ring Research.
- ↑ (December 2014) A heart that beats for 500 years: age-related changes in cardiac proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in Arctica islandica, the longest-living noncolonial animal. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 69 (12): 1448–61.
- ↑ (August 2016)Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science 353 (6300): 702–4.
- ↑ (August 2017) Extreme longevity in a deep-sea vestimentiferan tubeworm and its implications for the evolution of life history strategies. Die Naturwissenschaften 104 (7–8): 63.
- ↑ Timiras, Paola S. (2003) Physiological Basis of Ageing and Geriatrics. Informa Health Care. Template:ISBN. p. 26.
- ↑ Is there a 400 pound lobster out there?. howstuffworks (2007-07-05).
- ↑ (2005) Consider the Lobster and Other Essays. Little, Brown & Company. ISBN 978-0-316-15611-0. {{ safesubst:#invoke:Unsubst||date=__DATE__ |$B= }}
- ↑ (June 2004) Emerging area of aging research: long-lived animals with "negligible senescence". Annals of the New York Academy of Sciences 1019 (1): 518–20.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedWilliams1957 - ↑ (July 2003) The free-radical hypothesis of aging goes prokaryotic. Cellular and Molecular Life Sciences 60 (7): 1333–41.
- ↑ (June 2009) Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proceedings of the National Academy of Sciences of the United States of America 106 (24): 9761–6.
- ↑ (April 2012) Effects of BRCA1 and BRCA2 mutations on female fertility. Proceedings. Biological Sciences 279 (1732): 1389–95.
- ↑ 29.0 29.1 (2004). Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology 50 (5): 265–90.
- ↑ (2011). The reproductive-cell cycle theory of aging: an update. Experimental Gerontology 46 (2–3): 100–7.
- ↑ (September 2011) SIP-ing the elixir of youth. Cell 146 (6): 859–60.
- ↑ University of California San Diego (2018-12-26). UC San Diego Researchers Identify How Skin Ages, Loses Fat and Immunity. Press release.
- ↑ (January 2019) Age-Related Loss of Innate Immune Antimicrobial Function of Dermal Fat Is Mediated by Transforming Growth Factor Beta. Immunity 50 (1): 121–136.e5.
- ↑ (September 1981) DNA damage as the primary cause of aging. The Quarterly Review of Biology 56 (3): 279–303.
- ↑ (June 2014) Increased macromolecular damage due to oxidative stress in the neocortex and hippocampus of WNIN/Ob, a novel rat model of premature aging. Neuroscience 269: 256–64.
- ↑ (2011). A review and appraisal of the DNA damage theory of ageing. Mutation Research 728 (1–2): 12–22.
- ↑ (August 2010) Genetic, epigenetic and posttranslational mechanisms of aging. Biogerontology 11 (4): 387–99.
- ↑ (1986). Genetic instability as the primary cause of human aging. Experimental Gerontology 21 (4–5): 283–319.
- ↑ (2006) "Reliability Theory of Aging and Longevity", Handbook of the Biology of Aging. San Diego, CA: Academic Press, 3–42.
- ↑ (2013). Autophagy and ageing: implications for age-related neurodegenerative diseases. Essays in Biochemistry 55: 119–31.
- ↑ (2013). Chronic caloric restriction and exercise improve metabolic conditions of dietary-induced obese mice in autophagy correlated manner without involving AMPK. Journal of Diabetes Research 2013: 852754.
- ↑ (September 2020)Heterochromatin: an epigenetic point of view in aging. Experimental & Molecular Medicine 52 (9): 1466–1474.
- ↑ (1997-07-01)The heterochromatin loss model of aging. Experimental Gerontology 32 (4–5): 383–94.
- ↑ (July 2012) Global heterochromatin loss: a unifying theory of aging?. Epigenetics 7 (7): 680–8.
- ↑ (May 2015) The mechanism of ageing: primary role of transposable elements in genome disintegration. Cellular and Molecular Life Sciences 72 (10): 1839–47.
- ↑ (May 2018) Longevity and transposon defense, the case of termite reproductives. Proceedings of the National Academy of Sciences of the United States of America 115 (21): 5504–5509.
- ↑ (October 2017) The Piwi-piRNA pathway: road to immortality. Aging Cell 16 (5): 906–911.
- ↑ 48.0 48.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedBernstein book - ↑ (1990). The crosslinking theory of aging—added evidence. Experimental Gerontology 25 (2): 91–5.
- ↑ (February 2008) Mitochondrial dysfunction as a cause of ageing. Journal of Internal Medicine 263 (2): 167–78.
- ↑ (November 1981) The aging process. Proceedings of the National Academy of Sciences of the United States of America 78 (11): 7124–8.
- ↑ (October 2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metabolism 6 (4): 280–93.
- ↑ (August 2001) Does oxidative damage to DNA increase with age?. Proceedings of the National Academy of Sciences of the United States of America 98 (18): 10469–74.
- ↑ (March 2005) Peripheral lymphocyte 8-OHdG levels correlate with age-associated increase of tissue oxidative DNA damage in Sprague-Dawley rats. Protective effects of caloric restriction. Experimental Gerontology 40 (3): 181–8.
- ↑ (October 2000) Mitochondria, oxidative DNA damage, and aging. Journal of the American Aging Association 23 (4): 199–218.
- ↑ Vijg J. From DNA damage to mutations: All roads lead to aging. Ageing Res Rev. 2021 Mar 9;68:101316. doi: 10.1016/j.arr.2021.101316. Epub ahead of print. PMID 33711511
- ↑ Stephen Moss, Big ears: they really do grow as we age The Guardian, July 17, 2013. Retrieved August 18, 2021.
- ↑ 58.0 58.1 I. G. Gerasimov and Dmitry Yu Ignatov, Age Dynamics of Body Mass and Human Lifespan Journal of Evolutionary Biochemistry and Physiology 40(3) (2004):343–349. Retrieved August 18 2021.
- ↑ Infertility: Overview Institute for Quality and Efficiency in Health Care, March 25, 2015. Retrieved August 18, 2021.
- ↑ James G. Ryall, Jonathan D. Schertzer, and Gordon S. Lynch, Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness Biogerontology 9(4) (August 2008): 213–228. Retrieved August 18, 2021.
- ↑ Vinoth K. Ranganathan, Vlodek Siemionow, Vinod Sahgal, and Guang H. Yue, Effects of Aging on Hand Function Journal of the American Geriatrics Society 49(11) (November 2001):1478–1484. Retrieved August 18, 2021.
- ↑ Refractive Errors National Eye Institute. Retrieved August 18, 2021.
- ↑ Hearing Loss and Older Adults National Institute on Deafness and Other Communication Disorders. Retrieved August 18, 2021.
- ↑ Cataracts National Eye Institute. Retrieved August 18, 2021.
- ↑ L.P. Fried, C.M. Tangen, J. Walston, A.B. Newman, C. Hirsch, J. Gottdiener, T. Seeman, R. Tracy, W.J. Kop, G. Burke, and M.A. McBurnie, Frailty in older adults: evidence for a phenotype The Journals of Gerontology Series A, Biological Sciences and Medical Sciences 56(3) (March 2001): M146-156. Retrieved August 18, 2021.
- ↑ The top 10 causes of death World Health Organization, December 9, 2020. Retrieved August 18, 2021.
- ↑ Rolando T. Lazaro, Sandra G. Reina-Guerra, and Myla Quiben, Umphred's Neurological Rehabilitation (Mosby, 2019, ISBN 0323611176).
- ↑ K. Warner Schaie, Developmental Influences on Adult Intelligence (Oxford University Press, 2012, ISBN 0195386132).
- ↑ Ian Stuart-Hamilton, The Psychology of Ageing: An Introduction (Jessica Kingsley Publishers, 2012, ISBN 184905245X).
- ↑ Mediterranean diet associated with lower risk of early death in cardiovascular disease patients European Society of Cardiology, August 29, 2016. Retrieved August 18, 2021.
- ↑ Rhonda Rowland, Experts challenge study linking sleep, life span CNN, February 15, 2002. Retrieved August 19, 2021.
- ↑ (December 2012) Exercise and longevity. Maturitas 73 (4): 312–7.
- ↑ (1996) Physical Activity and Health. ISBN 978-1-4289-2794-0.
- ↑ (February 2012) Exercise, inflammation and aging. Aging and Disease 3 (1): 130–40.
- ↑ 75.0 75.1 (August 2016) Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 354: i3857.
- ↑ (2007-08-01)Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends in Cognitive Sciences 11 (8): 342–348.
- ↑ (October 2015) Epigenetics and Understanding the Impact of Social Determinants of Health. Pediatric Clinics of North America 62 (5): 1227–40.
- ↑ (April 2014) Protocol for a systematic review of the association between chronic stress during the life course and telomere length. Systematic Reviews 3 (40): 40.
- ↑ (October 2012) Cortisol serum levels in familial longevity and perceived age: the Leiden longevity study. Psychoneuroendocrinology 37 (10): 1669–75.
- ↑ (October 2013) The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults. Journal of the American College of Cardiology 62 (18): 1694–1701.
- ↑ (July 2010) Social relationships and mortality risk: a meta-analytic review. PLOS Medicine 7 (7): e1000316.
- ↑ Social ties are good for your health. stanford.edu.
- ↑ (2012) Handbook of religion and health, 2nd, New York: Oxford University Press, 476.
- ↑ Married Vs Single: What Science Says Is Better For Your Health. medicaldaily.com (2 April 2015).
- ↑ (July 2012) Meta-analysis of marital dissolution and mortality: reevaluating the intersection of gender and age. Social Science & Medicine 75 (1): 46–59.
- ↑ Judith Phillips, Kristine Ajrouch, and Sarah Hillcoat-Nallétamby, Key Concepts in Social Gerontology (SAGE Publications, 2010, ISBN 1412922720).
- ↑ Teresa L. Scheid and Eric R. Wright (eds.), A Handbook for the Study of Mental Health (Cambridge University Press, 2017, ISBN 9781316500965).
- ↑ Susan K. Whitbourne and Stacey B. Whitbourne, Adult Development and Aging (Wiley, 2020, ISBN 1119667453).
- ↑ John A. Vincent, Understanding generations: political economy and culture in an ageing society The British Journal of Sociology 56(4) (December 2005): 579–599. Retrieved August 18, 2021.
- ↑ 90.0 90.1 Jose Miguel Guzman, Ann Pawliczko, Sylvia Beales, Celia Till, and Ina Voelcker (ed.), Ageing in the Twenty-First Century: A Celebration and a Challenge (United Nations Population Fund, HelpAge International, 2012, ISBN 0897149815).
- ↑ Carolyn Gregoire, Here's Everything That's Wrong With Our 'Under 30' Obsession Huffington Post, February 25, 2014. Retrieved August 18, 2021.
- ↑ Korea - Birthday Celebrations Asian Info. Retrieved August 18, 2021.
- ↑ Serena Sabatini, Barbora Silarova, Anthony Martyr, Rachel Collins, Clive Ballard, Kaarin J. Anstey, Sarang Kim, and Linda Clare, Associations of Awareness of Age-Related Change With Emotional and Physical Well-being: A Systematic Review and Meta-analysis The Gerontologist 60(6) (August 2020): e477–e490. Retrieved August 18, 2021.
- ↑ Jutta Heckhausen, Developmental Regulation in Adulthood: Age-Normative and Sociostructural Constraints as Adaptive Challenges (Cambridge University Press, 2006, ISBN 0521027136).
- ↑ Paul B. Baltes and Margaret M. Baltes, Successful Aging (Cambridge University Press, 1993, ISBN 052143582X).
- ↑ J.W. Rowe and R.L. Kahn, Human aging: usual and successful Science 237(4811) (July 1987): 143–149. Retrieved August 18, 2021.
ReferencesISBN links support NWE through referral fees
- Baltes, Paul B., and Margaret M. Baltes. Successful Aging. Cambridge University Press, 1993. ISBN 052143582X
- Bouchard, Claude, Steven N. Blair, and William L. Haskell (eds.). Physical Activity and Health. Human Kinetics, 2012. ISBN 0736095411
- Guzman, Jose Miguel, Ann Pawliczko, Sylvia Beales, Celia Till, and Ina Voelcker (eds.). Ageing in the Twenty-First Century: A Celebration and a Challenge. United Nations Population Fund, HelpAge International, 2012. ISBN 0897149815
- Heckhausen, Jutta. Developmental Regulation in Adulthood: Age-Normative and Sociostructural Constraints as Adaptive Challenges. Cambridge University Press, 2006. ISBN 0521027136
- Lazaro, Rolando T., Sandra G. Reina-Guerra, and Myla Quiben. Umphred's Neurological Rehabilitation. Mosby, 2019. ISBN 0323611176
- McDonald, Roger B. Biology of Aging. Garland Science, 2019. ISBN 0815345674
- Phillips, Judith, Kristine Ajrouch, and Sarah Hillcoat-Nallétamby. Key Concepts in Social Gerontology. SAGE Publications, 2010. ISBN 1412922720
- Schaie, K. Warner. Developmental Influences on Adult Intelligence. Oxford University Press, 2012. ISBN 0195386132
- Scheid, Teresa L., and Eric R. Wright (eds.). A Handbook for the Study of Mental Health. Cambridge University Press, 2017. ISBN 9781316500965
- Stuart-Hamilton, Ian. The Psychology of Ageing: An Introduction. Jessica Kingsley Publishers, 2012. ISBN 184905245X
- Whitbourne, Susan K., and Stacey B. Whitbourne. Adult Development and Aging. Wiley, 2020. ISBN 1119667453
External links
All links retrieved
- Aging and Death in Folklore
- Global AgeWatch
- Ageing in the 21st Century HelpAge International
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.



