Difference between revisions of "Adenosine triphosphate" - New World Encyclopedia
Rick Swarts (talk | contribs) |
|||
| (24 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Ebapproved}}{{Copyedited}}{{Paid}}{{Approved}}{{Images OK}}{{Submitted}}{{Status}} |
| + | |||
<div> | <div> | ||
<!-- Here is a table of data; skip past it to edit the text. —> | <!-- Here is a table of data; skip past it to edit the text. —> | ||
<!-- Submit {{:subst:chembox_simple_organic}} to get this template or go to [[:Template:Chembox_simple_organic]]. —> | <!-- Submit {{:subst:chembox_simple_organic}} to get this template or go to [[:Template:Chembox_simple_organic]]. —> | ||
| − | {| align="right" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090; width: 320px;" | + | {|class="infobox" width="275" style="float:right;"margin:0 0 1em 1em;" align="right" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090; width: 320px;" |
! {{chembox header}}| '''Adenosine 5'-triphosphate''' | ! {{chembox header}}| '''Adenosine 5'-triphosphate''' | ||
|- | |- | ||
| Line 14: | Line 15: | ||
| '''ATP<br/>''' | | '''ATP<br/>''' | ||
|- | |- | ||
| − | | | + | | Chemical formula |
| C<sub>10</sub>H<sub>16</sub>N<sub>5</sub>O<sub>13</sub>P<sub>3</sub> | | C<sub>10</sub>H<sub>16</sub>N<sub>5</sub>O<sub>13</sub>P<sub>3</sub> | ||
|- | |- | ||
| Line 20: | Line 21: | ||
| 507.181 g mol<sup>-1</sup> | | 507.181 g mol<sup>-1</sup> | ||
|- | |- | ||
| − | | | + | | CAS registry number |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| 56-65-5 | | 56-65-5 | ||
| − | |- | + | |- "class=infobox" |
| − | |||
| − | |||
| − | |||
| − | |||
|} | |} | ||
</div> | </div> | ||
| − | + | '''Adenosine triphosphate''' ('''ATP''') is the chemical compound known in [[biochemistry]] as the "molecular currency" of intracellular [[energy]] transfer; that is, ATP is able to store and transport chemical energy within [[cell (biology)|cell]]s. All cells—both [[prokaryote|prokaryotic]], such as [[bacteria]], and [[eukaryote|eukaryotic]], such as with [[amoeba]], [[fungi]], [[plant]]s, and [[animal]]s—use ATP as the main molecule for carrying energy, and as the principal energy source for endergonic, or energy-requiring, reactions. | |
| − | '''Adenosine triphosphate''' ('''ATP''') is the | ||
| − | + | Living cells require energy to survive and function, and most of this energy comes either via radiant energy or from chemical energy tied up in interatomic bonds of nutrient molecules. When nutrient molecules, such as those derived from [[carbohydrate]]s and [[fat]]s, are oxidized by cells, a portion of the free energy released can be captured in the chemical bonds of ATP. ATP allows cells to store energy as chemical potential and to circulate and use this energy. Cells are constantly creating and circulating ATP, and when cells need energy, they "spend ATP," leading it to be commonly referred to as the '''energy currency''' of life. | |
| − | ATP also plays an important role in the synthesis of [[nucleic acid]]s | + | In addition to its energy-related function, ATP also plays an important role in the synthesis of [[nucleic acid]]s and further in signal transduction pathways in which it provides the phosphate for the protein-kinase reactions. |
| − | + | {{toc}} | |
| − | ATP | + | The ubiquitous presence of ATP in the cells of all [[life|living]] [[organism]]s provides support for the view that newer creations are built on the foundation of earlier creations, with ATP having appeared very early in the history of cellular life. The universal use of ATP likewise reflects the conservative nature of creation, where the same or similar metabolic processes and chemical compounds repeatedly occur, and it reflects a connectedness from the simplest organisms to humans. The intricate manner in which ATP is integrated in fundamental metabolic pathways also reveals the complex coordination required between the parts of living systems. |
==Chemical properties== | ==Chemical properties== | ||
| − | ATP consists of | + | ATP consists of adenosine and three attached [[phosphate]] groups (triphosphate). Adenosine itself is composed of two major molecular entities, [[adenine]] (a nitrogen-containing molecule) and [[ribose]] (a five-carbon sugar). [[Adenosine monophosphate]] (AMP) has one phosphate group attached to adenosine, and [[adenosine diphosphate]] (ADP) has two attached phosphate groups. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{| | {| | ||
| Line 69: | Line 46: | ||
|} | |} | ||
| − | The | + | The three linked phosphoryl groups, starting with that on AMP, are referred to as the alpha (α), beta (β), and gamma (γ) phosphates. These linked phosphate groups are the "business end" of the molecule, as ATP stores energy in the bonds between the phosphate groups. A molecule of ATP is sometimes written as A~P~P~P, with the "~" representing a bond that contains potential chemical energy. |
| − | + | ATP is extremely rich in chemical energy, in particular between the second and third phosphate groups. As these chemical bonds are broken (as ATP is converted into [[ADP]] and an inorganic phosphate) the energy release is -12 kCal / mole ''in vivo'' (inside a living cell), and -7.3 kCal / mole ''in vitro'' (in laboratory conditions). Such a relatively massive release of energy from a single chemical change with the whole cycle of charging and discharging the molecule integrated perfectly into the regular cellular metabolism is what makes ATP so valuable to all forms of life. The molecules can be charged up at one site and transported to another site for discharge, somewhat like a dry cell battery. | |
| − | ATP energy is | + | |
| + | ==Synthesis== | ||
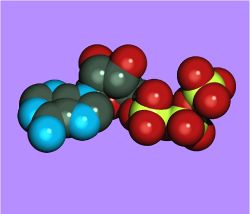
| + | [[Image:Atp_space_filling_ray_trace.jpg|250px|thumb|Space filling image of ATP]] | ||
| + | ATP can be produced by various cellular processes. Under aerobic conditions, the synthesis occurs in [[mitochondria]] during [[oxidative phosphorylation]], which is catalyzed by ATP synthase; to a lesser degree, under anaerobic conditions, this is done through substrate phosphorylation catalyzed by two enzymes: phosphoglycerate kinase (PGK) and pyruvate kinase. | ||
| − | + | ATP is also synthesized through several so-called "replenishment" reactions catalyzed by the enzyme families of NDKs (nucleoside diphosphate kinases), which use other nucleoside triphosphates as a high-energy phosphate donor, and the ATP guanido-phosphotransferase family, which uses [[creatine]]. | |
| − | |||
| − | + | ::[[adenosine diphosphate|ADP]] + [[guanosine triphosphate|GTP]] <math>\to</math> ATP + [[guanosine diphosphate|GDP]] | |
| − | |||
| − | + | In plants, ATP is synthesized in [[chloroplast]]s by [[photosynthesis]] during the light reactions of photosynthesis. However, this ATP is then used to power the [[Calvin cycle]] step of photosynthesis and so photosynthesis does not result in an overall production of ATP. | |
| − | + | The main fuels for ATP synthesis are [[glucose]] and [[fatty acid]]s. First, glucose is broken down into pyruvate in the cytosol yielding two molecules of ATP for each glucose molecule. Further breakdown of the glucose molecule for synthesizing ATP is carried out in the [[mitochondria]] in a process that yields about 30 molecules of ATP for each molecule of glucose that is oxidized. (See [[citric acid cycle]].) | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | ==Function== |
| + | ATP energy is released through [[hydrolysis]] (breakdown through reaction with water) of the high-energy phosphate-phosphate bonds. An enzyme, ATPase, aids in the breaking of the bond between the second and third phosphate groups, as ATP is converted to ADP. The hydrolysis yields free inorganic phosphate (P<sub>i</sub>) and [[adenosine diphosphate|ADP]]. Although this may result in free phosphate ions, usually the phosphate group is transferred to another molecule in a process called phosphorylation. | ||
| − | + | Energy is also released when the bond between the first and second phosphate groups is broken, as ADP is converted to AMP. That is, ADP can be broken down further to another P<sub>i</sub> and [[adenosine monophosphate|AMP]]. ATP can also be broken down to AMP directly, with the formation of pyrophosphate (PP<sub>i</sub>). This last reaction has the advantage of being an effectively irreversible process in aqueous solution. | |
| − | + | This energy can be used by a variety of [[enzyme]]s, motor [[protein]]s, and transport proteins to carry out the work of the cell. | |
| − | [[ | + | ==ATP in the human body== |
| + | The total quantity of ATP in the [[human body]] at any one time is about 0.1 [[Mole (unit)|mole]]. Yet, adults convert daily a quantity of ATP corresponding to at least half their body weight, and nearly a ton during a day of hard work. That is, the energy used by human cells requires the [[hydrolysis]] of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2,000 to 3,000 times during a single day. There is limited capacity to store ATP in a cell, and it is depleted in seconds, hence its consumption must closely follow its synthesis. That is, cells need to continually replenish or re-synthesize ATP. | ||
| − | + | ==References== | |
| − | + | *Abrahams, J.P., A. G. Leslie, R. Lutter, and J. E. Walker. 1994. Structure at 2.8 Å resolution of F 1 -ATPase from bovine heart mitochondria. ''Nature'' 370:621–628. | |
| − | + | *Boyer, P. D. 1993. The binding change mechanism for ATP synthase: Some probabilities and possibilities. ''Biochimica et Biophysica Acta'' 1140:215–250. | |
| − | + | *Boyer, P. D. 1997. The ATP synthase - a splendid molecular machine. ''Annual Review in Biochemistry'' 66:717–749. | |
| − | + | *Lutsenko, S., and J. H. Kaplan. 1996. Organization of P-type ATPases: Significance of structural diversity. ''Biochemistry'' 34:15607–15613. | |
| − | + | *Möller, J. V., B. Juul, and M. le Maire. 1996. Structural organization, ion transport, and energy transduction of P-type ATPases. ''Biochimica et Biophysica Acta'' 1286:1–51. | |
| − | + | *Skou, J. C. 1957. The influence of some cations on an adenosine triphosphatase from peripheral nerves. ''Biochimica et Biophysica Acta'' 23:394–401. | |
| − | + | *Skou, J. C., and M. Esmann. 1992. The Na, K-ATPase. ''Journal of Bioenergetics and Biomembranes'' 24:249–261. | |
| − | + | *Lingrel, J. B. 1992. Na-K-ATPase: Isoform structure, function, and expression. ''Journal of Bioenergetics and Biomembranes'' 24:263–270. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{{credit|32089683}} | {{credit|32089683}} | ||
| − | [[Category:Life sciences]] | + | [[Category:Life sciences]][[Category:Biochemistry]] |
Latest revision as of 21:33, 15 February 2016
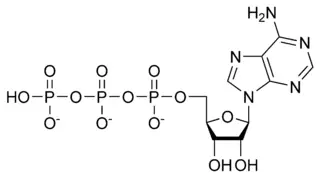
| Adenosine 5'-triphosphate | |
|---|---|
| |
| Chemical name |
[[[5-(6-aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl] methoxy-hydroxy-phosphoryl] oxy-hydroxy-phosphoryl] oxyphosphonic acid |
| Abbreviations | ATP |
| Chemical formula | C10H16N5O13P3 |
| Molecular mass | 507.181 g mol-1 |
| CAS registry number | 56-65-5 |
Adenosine triphosphate (ATP) is the chemical compound known in biochemistry as the "molecular currency" of intracellular energy transfer; that is, ATP is able to store and transport chemical energy within cells. All cells—both prokaryotic, such as bacteria, and eukaryotic, such as with amoeba, fungi, plants, and animals—use ATP as the main molecule for carrying energy, and as the principal energy source for endergonic, or energy-requiring, reactions.
Living cells require energy to survive and function, and most of this energy comes either via radiant energy or from chemical energy tied up in interatomic bonds of nutrient molecules. When nutrient molecules, such as those derived from carbohydrates and fats, are oxidized by cells, a portion of the free energy released can be captured in the chemical bonds of ATP. ATP allows cells to store energy as chemical potential and to circulate and use this energy. Cells are constantly creating and circulating ATP, and when cells need energy, they "spend ATP," leading it to be commonly referred to as the energy currency of life.
In addition to its energy-related function, ATP also plays an important role in the synthesis of nucleic acids and further in signal transduction pathways in which it provides the phosphate for the protein-kinase reactions.
The ubiquitous presence of ATP in the cells of all living organisms provides support for the view that newer creations are built on the foundation of earlier creations, with ATP having appeared very early in the history of cellular life. The universal use of ATP likewise reflects the conservative nature of creation, where the same or similar metabolic processes and chemical compounds repeatedly occur, and it reflects a connectedness from the simplest organisms to humans. The intricate manner in which ATP is integrated in fundamental metabolic pathways also reveals the complex coordination required between the parts of living systems.
Chemical properties
ATP consists of adenosine and three attached phosphate groups (triphosphate). Adenosine itself is composed of two major molecular entities, adenine (a nitrogen-containing molecule) and ribose (a five-carbon sugar). Adenosine monophosphate (AMP) has one phosphate group attached to adenosine, and adenosine diphosphate (ADP) has two attached phosphate groups.
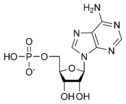 Adenosine monophosphate AMP |
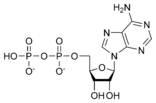 Adenosine diphosphate ADP |
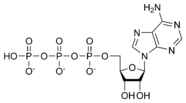 Adenosine triphosphate ATP |
The three linked phosphoryl groups, starting with that on AMP, are referred to as the alpha (α), beta (β), and gamma (γ) phosphates. These linked phosphate groups are the "business end" of the molecule, as ATP stores energy in the bonds between the phosphate groups. A molecule of ATP is sometimes written as A~P~P~P, with the "~" representing a bond that contains potential chemical energy.
ATP is extremely rich in chemical energy, in particular between the second and third phosphate groups. As these chemical bonds are broken (as ATP is converted into ADP and an inorganic phosphate) the energy release is -12 kCal / mole in vivo (inside a living cell), and -7.3 kCal / mole in vitro (in laboratory conditions). Such a relatively massive release of energy from a single chemical change with the whole cycle of charging and discharging the molecule integrated perfectly into the regular cellular metabolism is what makes ATP so valuable to all forms of life. The molecules can be charged up at one site and transported to another site for discharge, somewhat like a dry cell battery.
Synthesis
ATP can be produced by various cellular processes. Under aerobic conditions, the synthesis occurs in mitochondria during oxidative phosphorylation, which is catalyzed by ATP synthase; to a lesser degree, under anaerobic conditions, this is done through substrate phosphorylation catalyzed by two enzymes: phosphoglycerate kinase (PGK) and pyruvate kinase.
ATP is also synthesized through several so-called "replenishment" reactions catalyzed by the enzyme families of NDKs (nucleoside diphosphate kinases), which use other nucleoside triphosphates as a high-energy phosphate donor, and the ATP guanido-phosphotransferase family, which uses creatine.
- ADP + GTP ATP + GDP
In plants, ATP is synthesized in chloroplasts by photosynthesis during the light reactions of photosynthesis. However, this ATP is then used to power the Calvin cycle step of photosynthesis and so photosynthesis does not result in an overall production of ATP.
The main fuels for ATP synthesis are glucose and fatty acids. First, glucose is broken down into pyruvate in the cytosol yielding two molecules of ATP for each glucose molecule. Further breakdown of the glucose molecule for synthesizing ATP is carried out in the mitochondria in a process that yields about 30 molecules of ATP for each molecule of glucose that is oxidized. (See citric acid cycle.)
Function
ATP energy is released through hydrolysis (breakdown through reaction with water) of the high-energy phosphate-phosphate bonds. An enzyme, ATPase, aids in the breaking of the bond between the second and third phosphate groups, as ATP is converted to ADP. The hydrolysis yields free inorganic phosphate (Pi) and ADP. Although this may result in free phosphate ions, usually the phosphate group is transferred to another molecule in a process called phosphorylation.
Energy is also released when the bond between the first and second phosphate groups is broken, as ADP is converted to AMP. That is, ADP can be broken down further to another Pi and AMP. ATP can also be broken down to AMP directly, with the formation of pyrophosphate (PPi). This last reaction has the advantage of being an effectively irreversible process in aqueous solution.
This energy can be used by a variety of enzymes, motor proteins, and transport proteins to carry out the work of the cell.
ATP in the human body
The total quantity of ATP in the human body at any one time is about 0.1 mole. Yet, adults convert daily a quantity of ATP corresponding to at least half their body weight, and nearly a ton during a day of hard work. That is, the energy used by human cells requires the hydrolysis of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2,000 to 3,000 times during a single day. There is limited capacity to store ATP in a cell, and it is depleted in seconds, hence its consumption must closely follow its synthesis. That is, cells need to continually replenish or re-synthesize ATP.
ReferencesISBN links support NWE through referral fees
- Abrahams, J.P., A. G. Leslie, R. Lutter, and J. E. Walker. 1994. Structure at 2.8 Å resolution of F 1 -ATPase from bovine heart mitochondria. Nature 370:621–628.
- Boyer, P. D. 1993. The binding change mechanism for ATP synthase: Some probabilities and possibilities. Biochimica et Biophysica Acta 1140:215–250.
- Boyer, P. D. 1997. The ATP synthase - a splendid molecular machine. Annual Review in Biochemistry 66:717–749.
- Lutsenko, S., and J. H. Kaplan. 1996. Organization of P-type ATPases: Significance of structural diversity. Biochemistry 34:15607–15613.
- Möller, J. V., B. Juul, and M. le Maire. 1996. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochimica et Biophysica Acta 1286:1–51.
- Skou, J. C. 1957. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochimica et Biophysica Acta 23:394–401.
- Skou, J. C., and M. Esmann. 1992. The Na, K-ATPase. Journal of Bioenergetics and Biomembranes 24:249–261.
- Lingrel, J. B. 1992. Na-K-ATPase: Isoform structure, function, and expression. Journal of Bioenergetics and Biomembranes 24:263–270.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.