Difference between revisions of "Supercritical fluid" - New World Encyclopedia
(added credit and category tags, deleted foreign language links) |
|||
| Line 50: | Line 50: | ||
|} | |} | ||
² "Properties of Water and Steam", W. Wagner & A. Kruse. | ² "Properties of Water and Steam", W. Wagner & A. Kruse. | ||
| + | |||
| + | == Supercritical carbon dioxide == | ||
| + | |||
| + | '''Supercritical carbon dioxide''' refers to [[carbon dioxide]] with some unique properties. | ||
| + | Carbon dioxide usually behaves as a [[gas]] in air or as a [[solid]] in [[dry ice]]. If the [[temperature]] and [[pressure]] are both increased, it can adopt properties midway between a gas and a liquid. It behaves like a supercritical fluid above its [[critical temperature]] (31.1 degrees Celsius) and pressure (73 atm), expanding to fill its container like a gas, but with a density like that of a liquid. Supercritical CO<sub>2</sub> is becoming an important commercial and industrial solvent due to its role in compound extraction as well as its low toxicity and environmental impact. The relatively low temperature of the process and the stability of CO<sub>2</sub> also allows most compounds to be extracted with little damage or denaturing. | ||
| + | |||
| + | === Uses === | ||
| + | |||
| + | Supercritical carbon dioxide is gaining popularity amongst [[coffee]] manufacturers looking to move away from some of the classic decaffeinating [[solvents]] of the past; many of which have led to public outcry because of real or perceived dangers related to their use in food preparation. Supercritical CO<sub>2</sub> is forced through the green coffee beans and then sprayed with water at high pressure to remove the caffeine. The caffeine can then be isolated for resale to, for example, cola manufacturers by passing the water through [[activated charcoal filter]]s or by [[distillation]], [[crystallization]] or [[reverse osmosis]]. | ||
| + | |||
| + | Supercritical carbon dioxide is also becoming a more common process for extracting volatile oils and fragrance compounds from various raw materials that are used in [[perfume|perfumery]]. The relatively low critical temperature and reactivity of CO<sub>2</sub> allows the fragrance compounds to be extracted without extensive damage or denaturing, which will alter their [[odor]]. | ||
| + | |||
| + | Supercritical carbon dioxide can be used in cleaning clothes, instead of [[tetrachloroethylene]] (perchloroethylene or "perc") or water. This new approach of cleaning clothes was developed and commercialized by Dr. Joseph DeSimone, professor of chemistry at the [[University of North Carolina at Chapel Hill]]. | ||
| + | |||
| + | Processes which use supercritical [[carbon dioxide]] to produce micro and [[nano]] scale particles, often for [[pharmaceutical]] uses, are currently being developed. The gas antisolvent process, rapid expansion of supercritical solutions, and supercritical antisolvent precipitation (as well as several related methods) have been shown to process a variety of substances into particles [http://dx.doi.org/10.1016/j.supflu.2004.10.006 (Yeo and Kiran 2005)]. | ||
| + | |||
| + | Supercritical carbon dioxide is also used in the foaming of polymers. Many corporations utilize supercritical carbon dioxide to saturate the polymer with solvent (carbon dioxide). Upon depressurization and heating the carbon dioxide rapidly expands, causing voids within the polymer matrix, i.e. creating a foam. Research is also ongoing at many universities in the production of microcellular foams using supercritical carbon dioxide. | ||
| + | |||
| + | Supercritical carbon dioxide is beginning to be used to enhance oil recovery in mature oil fields. | ||
| + | |||
| + | === Environmental impact === | ||
| + | |||
| + | Supercritical carbon dioxide is seen as a promising green solvent because it is non-toxic, and a byproduct of other industrial processes. Furthermore, separation of the reaction components from the starting material is much simpler than with traditional [[organic solvent]]s. | ||
==See also== | ==See also== | ||
| − | *[[ | + | |
| + | *[[Caffeine]] | ||
| + | *[[Dry cleaning]] | ||
| + | *[[Fluid]] | ||
| + | *[[Perfume]] | ||
==References== | ==References== | ||
| − | + | ||
| − | + | * R.C. Reid, J.M. Prausnitz and B.E. Poling, ''The properties of gases and liquids,'' 4th ed., McGraw-Hill, New York, 1987. | |
| + | * W. Wagner and A. Kruse, ''Properties of Water and Steam'', Springer-Verlag, Berlin, 1998. | ||
| + | * Mukhopadhyay M. Natural extracts using supercritical carbon dioxide. USA: CRC Press, LLC; 2000; ISBN 0-8493-0819-4 | ||
| + | * [http://dx.doi.org/10.1016/j.supflu.2004.10.006 S. Yeo, E. Kiran, Formation of polymer particles with supercritical fluids: a review, J. Supercrit. Fluids 34 (2005) 287.] | ||
==Further reading== | ==Further reading== | ||
Revision as of 00:57, 2 December 2006
A supercritical fluid is any substance at a temperature and pressure above its thermodynamic critical point. It has the unique ability to diffuse through solids like a gas, and dissolve materials like a liquid. Additionally, it can readily change in density upon minor changes in temperature or pressure. These properties make it suitable as a substitute for organic solvents in a process called Supercritical Fluid Extraction. Carbon dioxide and water are the most commonly used supercritical fluids.
Introduction
In 1822, Baron Charles Cagniard de la Tour discovered the critical point of a substance in his famous cannon barrel experiments. Listening to discontinuities in the sound of a rolling flint ball in a sealed cannon filled with fluids at various temperatures, he observed the critical temperature. Above this temperature, the densities of the liquid and gas phases become equal and the distinction between them disappears, resulting in a single supercritical fluid phase. In Table 1, the critical properties are shown for some components, which are commonly used as supercritical fluids.
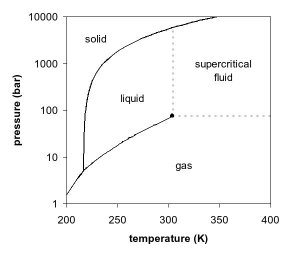
Phase diagram
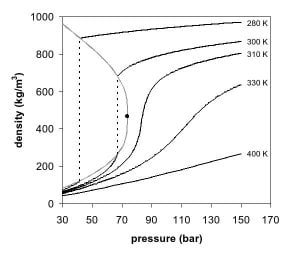
The observations by de la Tour can be explained by looking at the phase diagram of a pure component, e.g. carbon dioxide. In Figures 1 and 2, two projections of the phase diagram of carbon dioxide are shown. In the pressure-temperature phase diagram (Fig. 1) the boiling line is observed, which separates the vapor and liquid region and ends in the critical point. At the critical point, the densities of the equilibrium liquid phase and the saturated vapor phases become equal, resulting in the formation of a single supercritical phase. This can be observed in the density-pressure phase diagram for carbon dioxide, as shown in Figure 2, where the critical point is located at 304.1 K and 7.38 MPa (73.8 bar). With increasing temperatures, the liquid-vapor density gap decreases, up to the critical temperature, at which the discontinuity disappears. Thus, above the critical temperature a gas cannot be liquefied by pressure. However, at extremely high pressures the fluid can solidify, as visible at the top of Figure 1. By definition, a supercritical fluid is a substance above both its critical temperature and pressure. In a practical sense, the area of interest in supercritical fluids for processing and separation purposes is limited to temperatures in the vicinity of the critical point, where large gradients in the physical properties are observed. The changes near the critical point are not limited to density. Many other physical properties also show large gradients with pressure near the critical point, e.g. viscosity, the relative permittivity and the solvent strength, which are all closely related to the density. At higher temperatures, the fluid starts to behave like a gas, as can be seen in Figure 2. For carbon dioxide at 400 K, the density increases almost linearly with pressure.
Applications
For engineering purposes, supercritical fluids can be regarded as “hybrid solvents” with properties between those of gases and liquids, i.e. a solvent with a low viscosity, high diffusion rates and no surface tension. In the case of supercritical carbon dioxide, the viscosity is in the range of 20–100 µPa·s (0.02-0.1 cP), where liquids have viscosities of approximately 500–1000 µPa·s (0.5-1.0 cP) and gases approximately 10 µPa·s (0.01 cP), respectively. Diffusivities of solutes in supercritical carbon dioxide are up to a factor 10 higher than in liquid solvents. Additionally, these properties are strongly pressure-dependent in the vicinity of the critical point, making supercritical fluids highly tunable solvents. Of the components shown in Table 1, carbon dioxide and water are the most frequently used in a wide range of applications, including extractions, dry cleaning and chemical waste disposal. In polymer systems, ethylene and propylene are also widely used, where they act both as a solvent and as the reacting monomer.
| Solvent | Molecular Weight | Temperature | Pressure | Density | |
|---|---|---|---|---|---|
| Units | (g/mol) | (K) | (MPa) | (bar) | (g/cm³) |
| Carbon dioxide | 44.01 | 304.1 | 7.38 | 73.8 | 0.469 |
| Water | 18.02 | 647.3 (647.096)² | 22.12 (22.064)² | 221.2 (220.64)² | 0.348 |
| Methane | 16.04 | 190.4 | 4.60 | 46.0 | 0.162 |
| Ethane | 30.07 | 305.3 | 4.87 | 48.7 | 0.203 |
| Propane | 44.09 | 369.8 | 4.25 | 42.5 | 0.217 |
| Ethylene | 28.05 | 282.4 | 5.04 | 50.4 | 0.215 |
| Propylene | 42.08 | 364.9 | 4.60 | 46.0 | 0.232 |
| Methanol | 32.04 | 512.6 | 8.09 | 80.9 | 0.272 |
| Ethanol | 46.07 | 513.9 | 6.14 | 61.4 | 0.276 |
| Acetone | 58.08 | 508.1 | 4.70 | 47.0 | 0.278 |
² "Properties of Water and Steam", W. Wagner & A. Kruse.
Supercritical carbon dioxide
Supercritical carbon dioxide refers to carbon dioxide with some unique properties. Carbon dioxide usually behaves as a gas in air or as a solid in dry ice. If the temperature and pressure are both increased, it can adopt properties midway between a gas and a liquid. It behaves like a supercritical fluid above its critical temperature (31.1 degrees Celsius) and pressure (73 atm), expanding to fill its container like a gas, but with a density like that of a liquid. Supercritical CO2 is becoming an important commercial and industrial solvent due to its role in compound extraction as well as its low toxicity and environmental impact. The relatively low temperature of the process and the stability of CO2 also allows most compounds to be extracted with little damage or denaturing.
Uses
Supercritical carbon dioxide is gaining popularity amongst coffee manufacturers looking to move away from some of the classic decaffeinating solvents of the past; many of which have led to public outcry because of real or perceived dangers related to their use in food preparation. Supercritical CO2 is forced through the green coffee beans and then sprayed with water at high pressure to remove the caffeine. The caffeine can then be isolated for resale to, for example, cola manufacturers by passing the water through activated charcoal filters or by distillation, crystallization or reverse osmosis.
Supercritical carbon dioxide is also becoming a more common process for extracting volatile oils and fragrance compounds from various raw materials that are used in perfumery. The relatively low critical temperature and reactivity of CO2 allows the fragrance compounds to be extracted without extensive damage or denaturing, which will alter their odor.
Supercritical carbon dioxide can be used in cleaning clothes, instead of tetrachloroethylene (perchloroethylene or "perc") or water. This new approach of cleaning clothes was developed and commercialized by Dr. Joseph DeSimone, professor of chemistry at the University of North Carolina at Chapel Hill.
Processes which use supercritical carbon dioxide to produce micro and nano scale particles, often for pharmaceutical uses, are currently being developed. The gas antisolvent process, rapid expansion of supercritical solutions, and supercritical antisolvent precipitation (as well as several related methods) have been shown to process a variety of substances into particles (Yeo and Kiran 2005).
Supercritical carbon dioxide is also used in the foaming of polymers. Many corporations utilize supercritical carbon dioxide to saturate the polymer with solvent (carbon dioxide). Upon depressurization and heating the carbon dioxide rapidly expands, causing voids within the polymer matrix, i.e. creating a foam. Research is also ongoing at many universities in the production of microcellular foams using supercritical carbon dioxide.
Supercritical carbon dioxide is beginning to be used to enhance oil recovery in mature oil fields.
Environmental impact
Supercritical carbon dioxide is seen as a promising green solvent because it is non-toxic, and a byproduct of other industrial processes. Furthermore, separation of the reaction components from the starting material is much simpler than with traditional organic solvents.
See also
- Caffeine
- Dry cleaning
- Fluid
- Perfume
ReferencesISBN links support NWE through referral fees
- R.C. Reid, J.M. Prausnitz and B.E. Poling, The properties of gases and liquids, 4th ed., McGraw-Hill, New York, 1987.
- W. Wagner and A. Kruse, Properties of Water and Steam, Springer-Verlag, Berlin, 1998.
- Mukhopadhyay M. Natural extracts using supercritical carbon dioxide. USA: CRC Press, LLC; 2000; ISBN 0-8493-0819-4
- S. Yeo, E. Kiran, Formation of polymer particles with supercritical fluids: a review, J. Supercrit. Fluids 34 (2005) 287.
Further reading
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.