Difference between revisions of "Metabolic disease" - New World Encyclopedia
(→Mitochondrial disease: for now) |
(Mitochondrial disorders chart fixed) |
||
| Line 138: | Line 138: | ||
*weight loss | *weight loss | ||
|---- | |---- | ||
| − | |Myoclonic epilepsy and ragged-red fibers (MERRF) |*progressive myoclonic epilepsy | + | |Myoclonic epilepsy and ragged-red fibers (MERRF) |
| + | |*progressive myoclonic epilepsy | ||
*clumps of diseased mitochondria accumulate in the subsarcolemmal region of the muscle fiber and appear as "ragged-red fibers" when muscle is stained with modified Gomori trichrome stain | *clumps of diseased mitochondria accumulate in the subsarcolemmal region of the muscle fiber and appear as "ragged-red fibers" when muscle is stained with modified Gomori trichrome stain | ||
*short stature | *short stature | ||
|---- | |---- | ||
|Leigh syndrome, subacute sclerosing encephalopathy | |Leigh syndrome, subacute sclerosing encephalopathy | ||
| − | |*after normal development the disease usually begins late in the first year of life, but the onset may occur in adulthood | + | |*after normal development the disease usually begins late in the first year of life, but the onset may occur in adulthood |
| − | *a rapid decline in function occurs and is marked by seizures, altered states of consciousness, dementia, ventilatory failure | + | *a rapid decline in function occurs and is marked by seizures, altered states of consciousness, dementia, ventilatory failure |
|---- | |---- | ||
| − | |Neuropathy, ataxia, retinitis pigmentosa, and ptosis (NARP) |*progressive symptoms as described in the acronym | + | |Neuropathy, ataxia, retinitis pigmentosa, and ptosis (NARP) |
| + | |*progressive symptoms as described in the acronym | ||
*[[dementia]] | *[[dementia]] | ||
|---- | |---- | ||
| Line 154: | Line 156: | ||
*sensory-neural hearing loss | *sensory-neural hearing loss | ||
|---- | |---- | ||
| − | |Myoneurogenic gastrointestinal encephalopathy (MNGIE) |*gastrointestinal pseudo-obstruction | + | |Myoneurogenic gastrointestinal encephalopathy (MNGIE) |
| + | |*gastrointestinal pseudo-obstruction | ||
*[[neuropathy]] | *[[neuropathy]] | ||
|---- | |---- | ||
Revision as of 17:57, 2 August 2006
A metabolic disorder is any disease or disorder that affects the production or utilization of chemical energy from food (such as carbohydrates, proteins, and fats) within individual human cells. Although a few metabolic disorders are "acquired" as a result of diet, toxins, or infections, this article will focus on metabolic disorders with a genetic basis, which are also known as inborn errors of metabolism.
Cellular metabolism consists of numerous interconnected pathways that are catalyzed by enzymes in a series of stepwise biochemical reactions. Metabolic disorders typically result when an enzyme necessary for some step in a metabolic process is missing or improperly constructed due to a genetic defect. Depending on the enzyme’s function within the body, one of three major types of metabolic disorders may result:
- Disorders that give rise to toxic substances: the substrate typically catalyzed by the enzyme might accumulate to toxic levels.
- Disorders involving energy metabolism: an enzyme defective within a particular organ or tissue, such as the liver, muscle, or brain, might lead to low energy production or prevent transport to the part of the body requiring energy.
- Disorders of complex molecules: in the absence of a particular enzyme, the abnormal or unregulated synthesis of complex molecules might result. For example, in familial hypercholesterolemia, enzymes do not receive the signals that typically inhibit cholesterol synthesis, so that excessive production of cholesterol occurs, leading to early coronary vascular disease and strokes in patients.
Given the number of metabolic disorders and the range of systems affected, these disorders are manifested in a wide array of symptoms of varying severity, ranging from recurrent vomiting, lethargy, and muscle weakness, to liver and heart failure, developmental delay, and mental retardation (even within the same disorder, symptoms may vary, depending on the age of onset and other factors). Prenatal testing for some metabolic disorders using mass spectrometry is available and may result in earlier treatment and a better outcome; it is typically administered to families who are in a defined ethnic group in which the disorder has a relatively high incidence. Late onset of a metabolic disease is often triggered by acute metabolic stresses, such as infection, fasting, or consumption of a nutrient for which a metabolic intolerance exists. Therapies may include a restrictive diet, dietary supplements, and toxin-removal procedures, as well as enzyme replacement, gene transfer, or organ transplantation. Some severe diseases, such as many of the lipid storage diseases, currently have no effective therapy.
The genetics of metabolic disorders
From genes to enzymes
The instructions for building nearly all the enzymes involved in metabolism are stored as deoxyribonucleic acid (DNA) in the nucleus of the cell. In 1908, physician Sir Archibald Garrod coined the term “inborn errors of metabolism” to suggest that defects in specific biochemical pathways were due to an inadequate supply or a lack of a given enzyme. The link between enzymes involved in metabolism and genes was elaborated by geneticists George Beadle and Edward Tatum in 1945:
- All biochemical processes in all organisms are under genetic control.
- These biochemical processes can be broken down into a series of individual stepwise reactions.
- Each biochemical reaction is under the ultimate control of a different single gene.
- The mutation of a single gene results in an alternation in the ability of the cell to carry out a single primary chemical reaction.
Although this “one gene-one enzyme” principle has since been refined (not all gene products are enzymes, and some enzymes are composed of multiple units coded by different genes), it does suggest the following basic principle: inborn errors of metabolism are caused by mutant genes that produce abnormal enzymes whose function is altered.
Types of inheritance
Most metabolic disorders are inherited from one or both parents who carry a defective gene that regulates a particular protein in a class of the body’s cells. There are three primary types of inheritance involved in metabolic disorders:
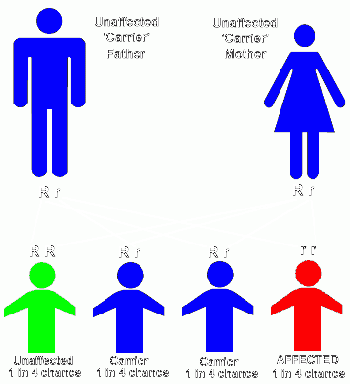
- Autosomal recessive inheritance occurs when both parents carry and pass on a copy of the faulty gene, but neither parent is affected by the disorder. Each child born to these parents has a 25% chance of inheriting both copies of the defective gene, a 50 % chance of being a carrier, and a 25% chance of not inheriting either copy of the defective gene. Children of either gender can be affected by an autosomal recessive pattern of inheritance.
- X-linked (or sex-linked) recessive inheritance occurs when the mother carries the affected gene on the X chromosome that determines the child’s gender and passes it to her son. Chromosomes are DNA-protein complexes that contain genetic material; females have two X chromosomes, while males have an X and a Y chromosome. Thus, sons of carriers have a 50% chance of inheriting the disorder, if the mutant gene is part of the X chromosome. Since daughters have two X chromosomes, they have a 50% chance of inheriting the X-linked chromosome but usually are not severely affected by the disorders. Affected men do not pass the disorder to their sons, but their daughters will be carriers for the disorder.
- Mitochondrial inheritance behaves differently from autosomal and sex-linked inheritance. Excepting sperm and egg cells, nuclear DNA contains two copies per cell. One copy is inherited from the father and the other from the mother. Mitochondria, however, contain their own DNA (typically between two and 10 copies), which are all inherited from the mother (for more detailed inheritance patterns, see mitochondrial genetics). If a cell contains a significant percentage of abnormal mitochondria, the cell and its surrounding tissue will exhibit impaired function. Not all of the enzymes and other components necessary for proper mitochondrial function are encoded in the mitochondrial DNA; defects in nuclear DNA may also play a role in some mitochondrial disorders.
Disorders that give rise to toxic substances
Some metabolic disorders result from the accumulation of toxic compounds due to a faulty or missing enzyme in a metabolic pathway; they include many disorders of amino acid and organic acid metabolism as well as sugar intolerances.
Disorders of intoxication typically share certain clinical similarities: a symptom-free period followed by “intoxication” that is acute (with symptoms such as vomiting, lethargy, coma, and liver failure) or chronic (characterized by progressive developmental decay or cardiac failure). The expression of the disorder is often late in onset and intermittent, and is diagnosed using plasma and urine amino-acid or organic-acid chromatography. Treatment may require removal of the toxin by special diets, exchange transfusion, peritoneal dialysis (a method of removing waste from the blood), or hemodialysis (to decrease the blood ammonia level).
Disorders of amino acid metabolism
Amino acids are organic molecules involved in the synthesis of proteins; they also participate in the synthesis of other crucial biological molecules such as neurotransmitters and hormones.
Phenylketonuria (PKU) results from the decreased activity of phenylalanine hydroxylase, an enzyme that converts the amino acid phenylalanine into tyrosine, which is a precursor of several important hormones as well as skin, hair, and eye pigments. This enzyme deficiency results in the build-up of phenylalanine in the blood, which in turn results in progressive developmental delay, behavioral disturbances, and seizures. Diet coke cans contain a warning label for PKU sufferers because phenylalanine is one of the components of aspartame, a sweetener used in carbonated soft drinks.
Other examples of disorders of amino metabolism that also involve elevated levels of an amino acid or its metabolites include classic (hepatorenal or type 1) tyrosinemia, homocystinuria, and non-ketonic hyperglycinemia.
Urea-cycle defects
Amino acids can be degraded into ammonia, carbon dioxide, and water. The ammonia component of amino acids is normally disposed of during the urea cycle, in which the nitrogen waste is incorporated into the urea (the primary solid component of urine) and excreted in the urine. A defect in any of the enzymes of the urea cycle leads to a toxic accumulation of ammonia in the blood, which in turn can lead to poor feeding, vomiting, lethargy, and possibly coma in a newborn, and, after recurrent, untreated episodes, to mental retardation and developmental impairment.
Organic acidemias
Organic acids are carbon-based compounds that appear at abnormally elevated levels when metabolic pathways involving specific enzymes are blocked. Organic acidemias are conditions characterized by the accumulation of organic acids in body tissues and fluids. Maple syrup urine disease (MSUD), a disorder common in the Mennonites of Pennsylvania, involves the accumulation of the amino acids leucine, isoleucine, and valine in the blood and urine (giving the urine a characteristic odor of maple syrup); the build-up leads to progressive neurological deterioration characterized by seizures, comas, and mental retardation. Other examples of organic acidemias include propionic academia and methylmalonic academia (MMA).
Sugar intolerances
Accumulation of simple sugars such as galactose and fructose, whose metabolism plays a role in many different pathways, may also occur due to enzyme deficiencies; for example:
- Galactosemia, which often manifests when milk feeding is started in infants, involves a breakdown in the metabolism of galactose, a sugar found in milk, resulting in an accumulation of galactose-1-phosphate that leads to lethargy, progressive liver dysfunction, kidney disease, and weight loss; if left untreated or treated belatedly, mental retardation may occur.
- Hereditary fructose intolerance (HFI) is caused by a deficiency in a liver enzyme that helps in the ingestion of fructose, a sugar common in fruits, table sugar (sucrose), and infant formulas.
Disorders involving energy metabolism
These disorders of energy metabolism are partly due to a deficiency in energy production or utilization resulting from a defect in liver, myocardium, muscle, or brain. Depending on the area involved, symptoms may include hypglycemia (low blood sugar), hyperlactacidemia (lactic acid build-up), muscular weakness, cardiomyopathy (heart failure), circulatory collapse, and malformations.
Glycogen storage disorders
Glycogen is the storage form of glucose, kept at the ready so that the brain, red blood cells, and the adrenal gland, which utilize glucose as a fuel, can depend on a constant supply when energy is needed. Glycogen is often stored in the liver and in muscle tissue; during normal metabolism, glycogen is broken down to glucose and released into the blood to be transported to a glucose-hungry area of the body. Glycogen storage disorders (GSDs) occur when enzymes involved in glycogen breakdown are blocked, so that the supply of glycogen remains in the liver and muscle. For example, in GSD type I (von Gierke disease), the last step in glucose release from the liver is defective, leading to hypoglycemia, which can be treated by continuous drip feedings of glucose or frequent feedings of cornstarch. Other types of GSDs are listed in the table below.
Fatty acid oxidation defects
The oxidation (or breakdown) of fatty acids for energy occurs in the mitochondria of liver cells. Before the fatty acids can be degraded, they must be converted to acyl CoA (a step called activation) and moved from the cytoplasm of the cell into the mitochondrion, a process that involves a carrier molecule, carnitine, which is synthesized in the body but may also be obtained in the diet or as a dietary supplement. Some fatty acid oxidation disorders arise through the dysfunction of carnitine transport enzymes. Fatty acid oxidation disorders may account for approximately 5-10 percent of cases of sudden infant death syndrome (SIDS). Some of the more common fatty acid oxidation diseases are listed in the table below.
Glycogen storage disorders
| GSD Type | Alternative name | Enzyme Deficiency |
| I | Von Gierke's disease | glucose-6-phosphatase |
| II | Pompe's disease | Acid maltase |
| III | Cori's disease or Forbe's disease | glycogen debrancher |
| IV | Anderson’s disease | glycogen branching enzyme |
| V | McArdle disease | muscle glycogen phosphorylase |
| VI | Hers’s disease | liver phosphorylase |
| VII | Tarui's disease | muscle phosphofructokinase |
| IX | phosphorylase kinase | |
| XI | Fanconi-Bickel disease | glucose transporter |
Mitochondrial disease
Mitochondrial diseases are a group of disorders relating to the mitochondria, the organelles in which the energy of food molecules is converted into the ATP that powers most cell functions.
The effects of mitochondrial disease can be quite varied, depending on the organ affected by the abnormal mitochondria. Since the distribution of defective DNA may vary from organ to organ within the body, the mutation that in one person may cause liver disease might in another person cause a brain disorder. In addition, the severity of the defect varies widely. Some minor defects cause only "exercise intolerance", with no serious illness or disability. Other defects can more severely affect the operation of the mitochondria and can cause severe body-wide impacts. As a general rule, mitochondrial diseases are most severe when the defective mitochondria are present in the muscles or nerves, because these contain the most energy-hungry cells of the body.
Although mitochondrial disease varies greatly in presentation from person to person, several major categories of the disease have been defined:
Mitochondrial disorders
| Mitochondrial disorder | Characteristics/Symptoms |
| Progressive external ophthalmoplegia (PEO) | progressive ophthalmoparesis is the cardinal feature |
| Leber hereditary optic neuropathy (LHON) | Visual loss beginning in young adulthood |
| Wolff-Parkinson-White syndrome | Multiple sclerosis-type disease |
| Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like syndrome (MELAS) | *varying degrees of cognitive impairment and dementia
|
| Myoclonic epilepsy and ragged-red fibers (MERRF) | *progressive myoclonic epilepsy
|
| Leigh syndrome, subacute sclerosing encephalopathy | *after normal development the disease usually begins late in the first year of life, but the onset may occur in adulthood
|
| Neuropathy, ataxia, retinitis pigmentosa, and ptosis (NARP) | *progressive symptoms as described in the acronym
|
| Kearns-Sayre syndrome (KSS) | *external ophthalmoplegia
|
| Myoneurogenic gastrointestinal encephalopathy (MNGIE) | *gastrointestinal pseudo-obstruction
|
Disorders involving complex molecules
These disorders disturb the synthesis (or catabolism) of complex molecules such as cholesterol; symptoms are often permanent, progressive, and not related to food intake.
Cholesterol synthesis
Cholesterol is a type of lipid with many biochemical roles in the body, including the building and maintenance of cell membranes. Familial hypercholesterolemia is caused by a deficiency of a receptor on the surface of cells in the liver and other organs, so that cholesterol remains in the blood rather than being moved into the cells. In addition, the enzymes involved in cholesterol synthesis do not receive feedback inhibition signaling them to cease synthesis, so that production of more cholesterol is induced. Lipids may become deposited in the walls of blood vessels, which can lead to [[atherosclerosis], an abnormal thickening and hardening of the walls of the arteries that is the principal cause of coronary heart disease and other forms of cardiovascular disease.
Lysosomal disorders
Lysosomes are organelles within the cell where the breakdown of various biological molecules, such as lipids and proteins, occurs. In lysosomal storage disorders, enzyme deficiencies or faulty activity of enzymes result in the accumulation of biological molecules that are normally degraded, causing the abnormal storage of complex molecules such as glycolipids, oligosaccharides, and glycoproteins. Symptoms vary depending on where in the body the storage occurs, though characteristics of many lysosomal storage disorders include coarsening of facial features, eye abnormalities, enlarged liver and spleen, and bone disease as well as neurological impairments. Most of these diseases do not have effective treatments. See the table below for some types of lysosomal disorders.
ReferencesISBN links support NWE through referral fees
- Fernandes, John, Jean-Marie Saudubray, and Georges van den Berghe. 2000. Inborn Metabolic Diseases: Diagnosis and Treatment, 3rd Edition. New York, NY: Springer.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Metabolic_disorder history
- Glycogen_storage-disease history
- List_of_fatty_acid_metabolism_disorders history
- Lipid_storage_disorder history
- Mitochondrial-disease history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.