Difference between revisions of "Corticosteroid" - New World Encyclopedia
Rick Swarts (talk | contribs) (added article from Wikipedia and credit/category tags) |
Rick Swarts (talk | contribs) |
||
| Line 1: | Line 1: | ||
[[Image:Corticosterone.svg|thumb|300px|[[Corticosterone]]]] | [[Image:Corticosterone.svg|thumb|300px|[[Corticosterone]]]] | ||
| + | '''Corticosteroid''' is any of a class of [[steroid hormone]]s that either are produced in the [[adrenal cortex]] or are synthetic analogues. Corticosteroids have similar chemical structures but are involved in a wide range of [[physiology|physiologic]] systems such as [[stress (medicine)|stress response]], [[immune system|immune response]] and regulation of [[inflammation]], [[carbohydrate]] [[metabolism]], [[protein]] [[catabolism]],[[fat metabolism]], retention of sodium in the [[kidney]]s, bone development, blood [[electrolyte]] levels, and behavior. They also are known as '''adrenocortical hormones''' and '''adrenocorticosteroids'''. | ||
| − | + | There are two main groups of corticosteroids: glucocorticoids, | |
| + | |||
| + | |||
| + | ==Overview== | ||
| + | |||
| + | There are two main groups of corticosteroids. | ||
* '''[[Glucocorticoids]]''' such as [[cortisol]] control carbohydrate, fat and protein metabolism and are anti-inflammatory by preventing [[phospholipid]] release, decreasing [[eosinophil granulocyte|eosinophil]] action and a number of other mechanisms. | * '''[[Glucocorticoids]]''' such as [[cortisol]] control carbohydrate, fat and protein metabolism and are anti-inflammatory by preventing [[phospholipid]] release, decreasing [[eosinophil granulocyte|eosinophil]] action and a number of other mechanisms. | ||
Revision as of 21:22, 24 January 2009
Corticosteroid is any of a class of steroid hormones that either are produced in the adrenal cortex or are synthetic analogues. Corticosteroids have similar chemical structures but are involved in a wide range of physiologic systems such as stress response, immune response and regulation of inflammation, carbohydrate metabolism, protein catabolism,fat metabolism, retention of sodium in the kidneys, bone development, blood electrolyte levels, and behavior. They also are known as adrenocortical hormones and adrenocorticosteroids.
There are two main groups of corticosteroids: glucocorticoids,
Overview
There are two main groups of corticosteroids.
- Glucocorticoids such as cortisol control carbohydrate, fat and protein metabolism and are anti-inflammatory by preventing phospholipid release, decreasing eosinophil action and a number of other mechanisms.
- Mineralocorticoids such as aldosterone control electrolyte and water levels, mainly by promoting sodium retention in the kidney.
Some common natural hormones are corticosterone (C21H30O4), cortisone (C21H28O5, 17-hydroxy-11-dehydrocorticosterone) and aldosterone.
Biosynthesis
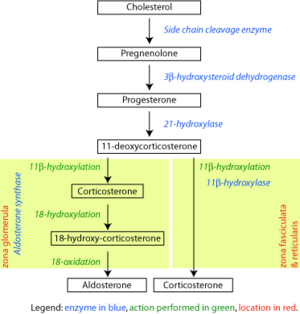
The corticosteroids are synthesized from cholesterol within the adrenal cortex. Most steroidogenic reactions are catalysed by enzymes of the cytochrome P450 family. They are located within the mitochondria and require adrenodoxin as a cofactor (except 21-hydroxylase and 17α-hydroxylase).
Aldosterone and corticosterone share the first part of their biosynthetic pathway. The last part is either mediated by the aldosterone synthase (for aldosterone) or by the 11β-hydroxylase (for corticosterone). These enzymes are nearly identical (they share 11β-hydroxylation and 18-hydroxylation functions). But aldosterone synthase is also able to perform an 18-oxidation. Moreover, aldosterone synthase is found within the zona glomerulosa at the outer edge of the adrenal cortex; 11β-hydroxylase is found in the zona fasciculata and reticularis.
Classes of corticosteroids
Corticosteroids are generally grouped into four classes, based on chemical structure. Allergic reactions to one member of a class typically indicate an intolerance of all members of the class.
The highlighted steroids are often used in the screening of allergies to topical steroids.[1]
Group A
(short to medium acting glucocorticoids) Hydrocortisone, Hydrocortisone acetate, Cortisone acetate, Tixocortol pivalate, Prednisolone, Methyprednisolone, and Prednisone.
Group B
Triamcinolone acetonide, Triamcinolone alcohol, Mometasone, Amcinonide, Budesonide, Desonide, Fluocinonide, Fluocinolone acetonide, and Halcinonide.
Group C
Betamethasone, Betamethasone sodium phosphate, Dexamethasone, Dexamethasone sodium phosphate, and Fluocortolone.
Group D
Hydrocortisone-17-butyrate, Hydrocortisone-17-valerate, Aclometasone dipropionate, Betamethasone valerate, Betamethasone dipropionate, Prednicarbate, Clobetasone-17-butyrate, Clobetasol-17-propionate, Fluocortolone caproate, Fluocortolone pivalate, and Fluprednidene acetate.
Types of Corticosteroids
1. Topical steroid for use topically on the skin, eye, and mucous membranes.
2. Inhaled steroids for use to treat the nasal mucosa, sinuses, bronchii, and lungs.[2]
3. Oral forms - such as prednisone and prednisolone.[3]
4. Systemic forms - available in injectibles for use intravenously and parenteral routes.[4]
Uses
Synthetic drugs with corticosteroid-like effect are used in a variety of conditions, ranging from brain tumors to skin diseases. Dexamethasone and its derivatives are almost pure glucocorticoids, while prednisone and its derivatives have some mineralocorticoid action in addition to the glucocorticoid effect. Fludrocortisone (Florinef) is a synthetic mineralocorticoid. Hydrocortisone (cortisol) is available for replacement therapy, e.g. in adrenal insufficiency and congenital adrenal hyperplasia.
Synthetic glucocorticoids are used in the treatment of joint pain or inflammation (arthritis), temporal arteritis, dermatitis, allergic reactions, asthma, hepatitis, systemic lupus erythematosus, inflammatory bowel disease (ulcerative colitis and Crohn's disease), sarcoidosis and for glucocorticoid replacement in Addison's disease or other forms of adrenal insufficiency. Topical formulations are also available for the skin, eyes, lungs, nose, and bowels. Corticosteroids are also used supportively to prevent nausea, often in combination with 5-HT3 antagonists (e.g. ondansetron).
Typical undesired effects of glucocorticoids present quite uniformly as drug-induced Cushing's syndrome. Typical mineralocorticoid side effects are hypertension (abnormally high blood pressure), hypokalemia (low potassium levels in the blood), hypernatremia (high sodium levels in the blood) without causing peripheral edema, metabolic alkalosis and connective tissue weakness (Werner, 2005).
Clinical and experimental evidence indicates that corticosteroids can cause permanent eye damage by inducing central serous retinopathy (CSR, also known as central serous chorioretinopathy, CSC). A variety of steroid medications, from anti-allergy nasal sprays (Nasonex, Flonase) to topical skin creams, to eye drops (Tobradex), to Prednisone have been implicated in the development of CSR.
History
Tadeusz Reichstein together with Edward Calvin Kendall and Philip Showalter Hench were awarded the Nobel Prize for Physiology and Medicine in 1950 for their work on hormones of the adrenal cortex which culminated in the isolation of cortisone.
Corticosteroids have been used as drug treatment for some time. Lewis Sarett of Merck & Co. was the first to synthesize cortisone, using a complicated 36-step process that started with deoxycholic acid, which was extracted from ox bile. The low efficiency of converting deoxycholic acid into cortisone led to a cost of US $200 per gram. Russell Marker, at Syntex, discovered a much cheaper and more convenient starting material, diosgenin from wild Mexican yams. His conversion of diosgenin into progesterone by a four-step process now known as Marker degradation was an important step in mass production of all steroidal hormones, including cortisone and chemicals used in hormonal contraception. In 1952, D.H. Peterson and H.C. Murray of Upjohn Co. developed a process that used Rhizopus mold to oxidize progesterone into a compound that was readily converted to cortisone. The ability to cheaply synthesize large quantities of cortisone from the diosgenin in yams resulted in a rapid drop in price to US $6 per gram, falling to $0.46 per gram by 1980. Percy Julian's research also aided progress in the field. The exact nature of cortisone's anti-inflammatory nature remained a mystery for years after however, until the leukocyte adhesion cascade and the role of phospholipase A2 in the production of prostaglandins and leukotrienes was fully understood in the early 1980s.
ReferencesISBN links support NWE through referral fees
Werner R (2005). A massage therapist's guide to Pathology. 3rd edition. Lippincott Williams & Wilkins, Pennsylvania, USA.
See also
- Cushing's syndrome
- Addison's disease
- Vitiligo
- Steroids (general term)
- Fluorometholone
- List of steroid abbreviations
- Topical steroid
References
- ↑ Wolverton, SE. Comprehensive Dermatologic Drug Therapy. WB Saunders, 2001. p. 562
- ↑ http://www.webmd.com/asthma/guide/asthma_control_with_anti-inflammatory-drugs
- ↑ http://dermnetnz.org/treatments/systemic-steroids.html
- ↑ http://dermnetnz.org/treatments/systemic-steroids.html
| |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.