Difference between revisions of "Aspartic acid" - New World Encyclopedia
({{Paid}}) |
m (Protected "Aspartic acid": copyedited [edit=sysop:move=sysop]) |
||
| Line 1: | Line 1: | ||
| − | {{Paid}}{{Approved}}{{Images OK}}{{Submitted}}{{Claimed}}{{Contracted}} | + | {{Paid}}{{Approved}}{{Images OK}}{{Submitted}}{{Claimed}}{{Contracted}}{{copyedited}} |
{{NatOrganicBox | {{NatOrganicBox | ||
| image= [[Image:L-aspartic-acid-skeletal.png|100px|Chemical structure of Aspartic acid]][[Image:L-aspartic-acid-3D-sticks.png|90px|Chemical structure of the amino acid aspartate]]<br />Chemical structure of L-aspartic acid | | image= [[Image:L-aspartic-acid-skeletal.png|100px|Chemical structure of Aspartic acid]][[Image:L-aspartic-acid-3D-sticks.png|90px|Chemical structure of the amino acid aspartate]]<br />Chemical structure of L-aspartic acid | ||
| Line 10: | Line 10: | ||
}} | }} | ||
| − | '''Aspartic acid''' | + | '''Aspartic acid,''' also called '''asparaginic acid''' and '''alpha-aminosuccinic acid,''' is an acidic, α-[[amino acid]] that is found in many [[protein]]s and is common in young [[sugar cane]] and [[sugar beet]]s. It is closely related to the amino acid [[asparagine]]. Along with [[glutamic acid]], it is classified as an acidic amino acid. |
| − | In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] required for normal functioning. However, it is considered to be [[amino acid#essential amino acid|non-essential]] since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. | + | In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] required for normal functioning. However, it is considered to be [[amino acid#essential amino acid|non-essential]], since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. |
Aspartic acid is pervasive in biosynthesis and is the precursor to several amino acids. Aspartic acid is a metabolite in the [[urea cycle]] and participates in [[gluconeogenesis]]. It also acts as a [[neurotransmitter]]. The non-[[carbohydrate]], non-nutritive artificial sweetener and flavor enhancer [[aspartame]] (aspartyl-phenylalanine-1-methyl ester) is synthesized from aspartic acid and the essential amino acid, [[phenylalanine]]. | Aspartic acid is pervasive in biosynthesis and is the precursor to several amino acids. Aspartic acid is a metabolite in the [[urea cycle]] and participates in [[gluconeogenesis]]. It also acts as a [[neurotransmitter]]. The non-[[carbohydrate]], non-nutritive artificial sweetener and flavor enhancer [[aspartame]] (aspartyl-phenylalanine-1-methyl ester) is synthesized from aspartic acid and the essential amino acid, [[phenylalanine]]. | ||
| − | The discovery, manufacture, and use of the sweetener aspartame, which is now found in many products, represents an aspect of human creativity, addressing a human desire for sweet things while trying to avoid the | + | The discovery, manufacture, and use of the sweetener aspartame, which is now found in many products, represents an aspect of human creativity, addressing a human desire for sweet things while trying to avoid the negative consequences traced to overconsumption of sugar. However, human [[creativity]] can be for good or bad, and some health risks have been alleged for aspartame. |
Aspartic acid's three letter code is ASP, its one letter code is D, its codons are GAU and GAC, and its systematic name is 2-Aminobutanedioic acid (IUPAC-IUB 1983). | Aspartic acid's three letter code is ASP, its one letter code is D, its codons are GAU and GAC, and its systematic name is 2-Aminobutanedioic acid (IUPAC-IUB 1983). | ||
==Structure== | ==Structure== | ||
| − | In [[biochemistry]], the term [[amino acid]] is frequently used to refer specifically to | + | In [[biochemistry]], the term [[amino acid]] is frequently used to refer specifically to alpha amino acids: Those amino acids in which the amino and [[carboxylate]] groups are attached to the same [[carbon]], the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is: |
''R'' | ''R'' | ||
| Line 30: | Line 30: | ||
where ''R'' represents a ''side chain'' specific to each amino acid. | where ''R'' represents a ''side chain'' specific to each amino acid. | ||
| − | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In aspartic acid, only the L-stereoisomer is involved in protein synthesis. | + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called [[proteinogenic amino acids]]. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In aspartic acid, only the L-stereoisomer is involved in protein synthesis. |
Aspartic acids chemical formula is HOOC-CH(NH<sub>2</sub>)-CH<sub>2</sub>-COOH, or more generally C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>. | Aspartic acids chemical formula is HOOC-CH(NH<sub>2</sub>)-CH<sub>2</sub>-COOH, or more generally C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>. | ||
| Line 40: | Line 40: | ||
==Biochemical role and uses== | ==Biochemical role and uses== | ||
| − | Aspartic acid is non-essential in [[mammal]]s, being produced from [[oxaloacetate]] by [[transamination]]. In plants and microorganisms, aspartic acid is the precursor to several amino acids, including four that are essential: [[ | + | Aspartic acid is non-essential in [[mammal]]s, being produced from [[oxaloacetate]] by [[transamination]]. In plants and microorganisms, aspartic acid is the precursor to several amino acids, including four that are essential: [[Methionine]], [[threonine]], [[isoleucine]], and [[lysine]]. The conversion of aspartic acid to these other amino acids begins with reduction of aspartic acid to its "semialdehyde," HO<sub>2</sub>CCH(NH<sub>2</sub>)CH<sub>2</sub>CHO (Lehninger et al. 2000). |
[[Asparagine]] is derived from aspartic acid via transamidation: | [[Asparagine]] is derived from aspartic acid via transamidation: | ||
| Line 46: | Line 46: | ||
(where ''G''C(O)NH<sub>2</sub> and ''G''C(O)OH are [[glutamine]] and [[glutamic acid]], respectively) | (where ''G''C(O)NH<sub>2</sub> and ''G''C(O)OH are [[glutamine]] and [[glutamic acid]], respectively) | ||
| − | Aspartic acid also is a metabolite (intermediates and products of [[metabolism]]) in the [[urea cycle]] and participates in [[gluconeogenesis]]. Gluconeogenesis is generation of [[glucose]] from non-sugar carbon substrates like pyruvate, lactate, glycerol, and glucogenic amino acids (primarily [[alanine]] and [[glutamine]]). | + | Aspartic acid also is a metabolite (intermediates and products of [[metabolism]]) in the [[urea cycle]] and participates in [[gluconeogenesis]]. Gluconeogenesis is the generation of [[glucose]] from non-sugar carbon substrates like pyruvate, lactate, glycerol, and glucogenic amino acids (primarily [[alanine]] and [[glutamine]]). |
Aspartic acid carries reducing equivalents in the [[malate-aspartate shuttle]], which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartic acid donates one nitrogen atom in the biosynthesis of [[inositol]], the precursor to the [[purine]] bases. | Aspartic acid carries reducing equivalents in the [[malate-aspartate shuttle]], which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartic acid donates one nitrogen atom in the biosynthesis of [[inositol]], the precursor to the [[purine]] bases. | ||
| Line 54: | Line 54: | ||
As a neurotransmitter, aspartic acid may provide resistance to fatigue and thus lead to endurance, although the evidence to support this idea is not strong. | As a neurotransmitter, aspartic acid may provide resistance to fatigue and thus lead to endurance, although the evidence to support this idea is not strong. | ||
| − | The artificial | + | The artificial sweetener and flavor enhancer, [[aspartame]] is made from aspartic acid and [[phenylalanine]]. It is made only from the L-isomers of the amino acids. Although L-aspartic acid has a flat taste and L-phenylalanine has a bitter taste, these can be combined with some modifications to give the sweet taste of aspartame. |
==References== | ==References== | ||
* Chen, P. E., M. T. Geballe, P. J. Stansfeld, A. R. Johnston, H. Yuan, A. L. Jacob, J. P. Snyder, S. F. Traynelis, and D. J. A. Wyllie. 2005. [http://molpharm.aspetjournals.org/cgi/content/full/67/5/1470 Structural features of the glutamate binding site in recombinant NR1/NR2A N-Methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling]. ''Molecular Pharmacology'' 67: 1470-1484. | * Chen, P. E., M. T. Geballe, P. J. Stansfeld, A. R. Johnston, H. Yuan, A. L. Jacob, J. P. Snyder, S. F. Traynelis, and D. J. A. Wyllie. 2005. [http://molpharm.aspetjournals.org/cgi/content/full/67/5/1470 Structural features of the glutamate binding site in recombinant NR1/NR2A N-Methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling]. ''Molecular Pharmacology'' 67: 1470-1484. | ||
| − | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319 | + | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319 |
* Dunn, M. S., and B. W. Smart. 1963. [http://www.orgsyn.org/orgsyn/pdfs/CV4P0055.pdf DL-Aspartic Acid]. ''Organic Syntheses'' 4: 55. | * Dunn, M. S., and B. W. Smart. 1963. [http://www.orgsyn.org/orgsyn/pdfs/CV4P0055.pdf DL-Aspartic Acid]. ''Organic Syntheses'' 4: 55. | ||
* International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | ||
| − | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry'' | + | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry,'' 3rd ed. New York: Worth Publishing. ISBN 1572591536 |
| Line 68: | Line 68: | ||
| − | |||
{{credit|Aspartic_acid|136970651|Gluconeogenesis|137415567}} | {{credit|Aspartic_acid|136970651|Gluconeogenesis|137415567}} | ||
[[Category:Life sciences]] | [[Category:Life sciences]] | ||
Revision as of 17:39, 1 November 2007
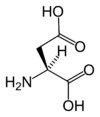 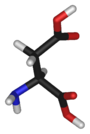 Chemical structure of L-aspartic acid | |
Aspartic acid | |
| Systematic (IUPAC) name | |
| (2S)-2-aminobutanedioic acid | |
| Identifiers | |
| CAS number | 56-84-8 |
| PubChem | 5960 |
| Chemical data | |
| Formula | C4H7NO4 |
| Mol. weight | 133.10 |
| SMILES | N[C@@H](CC(O)=O)C(O)=O |
| Complete data | |
Aspartic acid, also called asparaginic acid and alpha-aminosuccinic acid, is an acidic, α-amino acid that is found in many proteins and is common in young sugar cane and sugar beets. It is closely related to the amino acid asparagine. Along with glutamic acid, it is classified as an acidic amino acid.
In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids required for normal functioning. However, it is considered to be non-essential, since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions.
Aspartic acid is pervasive in biosynthesis and is the precursor to several amino acids. Aspartic acid is a metabolite in the urea cycle and participates in gluconeogenesis. It also acts as a neurotransmitter. The non-carbohydrate, non-nutritive artificial sweetener and flavor enhancer aspartame (aspartyl-phenylalanine-1-methyl ester) is synthesized from aspartic acid and the essential amino acid, phenylalanine.
The discovery, manufacture, and use of the sweetener aspartame, which is now found in many products, represents an aspect of human creativity, addressing a human desire for sweet things while trying to avoid the negative consequences traced to overconsumption of sugar. However, human creativity can be for good or bad, and some health risks have been alleged for aspartame.
Aspartic acid's three letter code is ASP, its one letter code is D, its codons are GAU and GAC, and its systematic name is 2-Aminobutanedioic acid (IUPAC-IUB 1983).
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: Those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In aspartic acid, only the L-stereoisomer is involved in protein synthesis.
Aspartic acids chemical formula is HOOC-CH(NH2)-CH2-COOH, or more generally C4H7NO4.
Aspartic acid behaves similarly to glutamic acid. It carries a hydrophilic acidic group with strong negative charge. Aspartic acid usually is located on the outer surface of the protein, making it water-soluble. It binds to positively-charged molecules and ions, often used in enzymes to fix the metal ion.
Synthesis
Racemic aspartic acid (equal amounts of left- and right-handed stereoisomers) can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2) (Dunn and Smart 1963).
Biochemical role and uses
Aspartic acid is non-essential in mammals, being produced from oxaloacetate by transamination. In plants and microorganisms, aspartic acid is the precursor to several amino acids, including four that are essential: Methionine, threonine, isoleucine, and lysine. The conversion of aspartic acid to these other amino acids begins with reduction of aspartic acid to its "semialdehyde," HO2CCH(NH2)CH2CHO (Lehninger et al. 2000).
Asparagine is derived from aspartic acid via transamidation:
- HO2CCH(NH2)CH2CO2H + GC(O)NH2 HO2CCH(NH2)CH2CONH2 + GC(O)OH
(where GC(O)NH2 and GC(O)OH are glutamine and glutamic acid, respectively)
Aspartic acid also is a metabolite (intermediates and products of metabolism) in the urea cycle and participates in gluconeogenesis. Gluconeogenesis is the generation of glucose from non-sugar carbon substrates like pyruvate, lactate, glycerol, and glucogenic amino acids (primarily alanine and glutamine).
Aspartic acid carries reducing equivalents in the malate-aspartate shuttle, which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartic acid donates one nitrogen atom in the biosynthesis of inositol, the precursor to the purine bases.
As a neurotransmitter, aspartate (the conjugate base of aspartic acid) stimulates NMDA receptors, though not as strongly as the amino acid neurotransmitter glutamate does (Chen et al. 2005). It serves as an excitatory neurotransmitter in the brain and is an excitotoxin.
As a neurotransmitter, aspartic acid may provide resistance to fatigue and thus lead to endurance, although the evidence to support this idea is not strong.
The artificial sweetener and flavor enhancer, aspartame is made from aspartic acid and phenylalanine. It is made only from the L-isomers of the amino acids. Although L-aspartic acid has a flat taste and L-phenylalanine has a bitter taste, these can be combined with some modifications to give the sweet taste of aspartame.
ReferencesISBN links support NWE through referral fees
- Chen, P. E., M. T. Geballe, P. J. Stansfeld, A. R. Johnston, H. Yuan, A. L. Jacob, J. P. Snyder, S. F. Traynelis, and D. J. A. Wyllie. 2005. Structural features of the glutamate binding site in recombinant NR1/NR2A N-Methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling. Molecular Pharmacology 67: 1470-1484.
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319
- Dunn, M. S., and B. W. Smart. 1963. DL-Aspartic Acid. Organic Syntheses 4: 55.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.