Lysine
| Lysine | |
|---|---|
|
|
| |
| IUPAC name | 2,6-diaminohexanoic acid |
| Other names | Lys, K |
| Identifiers | |
| CAS number | [] |
| PubChem | |
| EINECS number | |
| MeSH | |
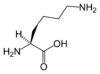
| SMILES | C(CCN)CC(C(=O)O)N |
| Properties | |
| Molecular formula | C6H14N2O2 |
| Molar mass | 146.188 |
| Melting point |
224 °C |
| Acidity (pKa) | 2.15, 9.16, 10.67 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Lysine is an α-amino acid that is present in many proteins but whose concentration is low in some plant proteins. In humans, the L-isomer of lysine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Valine is also classified as an "essential amino acid" since it cannot be synthesized by the human body from other compounds through chemical reactions and thus has to be taken in with the diet.
Lysine is of particular dietary importance since many cereal grains and vegetables are low in concentration of this amino acid or is not fully biologically available. Diets poor in the lysine, such as one based on grains, can cause deficiency of lysine, which will slow down protein synthesis and result in the body not being able to sustain growth and repair of muscle tissue (Longe 2006).
In the case of essential amino acids, it is important for individuals to have disciplined eating habits in order to get proper amounts. Means have been developed to synthesize lysine commerically and it is often a supplement to bread, rice, and cereal-based animal feeds (Bender and Bender 2005).
Lysine's three letter code is Lys, its one letter code is K, its codons are AAA and AAG, and its systematic name is 2,6-diaminohexanoic acid.
Structure
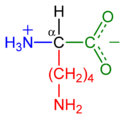
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In valine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Valine's chemical formula is (CH3)2CH-CH(NH2)-COOH, or in general form C5H11NO2 (IUPAC-IUB 1983).
Like isoleucine and leucine, valine has large aliphatic hydrophobic side chains. Its molecules are rigid, and its mutual hydrophobic interactions are important for the correct folding of proteins, as these chains tend to be located inside of the protein molecule.
Lysine is a basic, as are arginine and histidine. The ε-amino group often participates in hydrogen bonding and as a general base in catalysis.
Common posttranslational modifications include methylation of the e-amino group, giving methyl-, dimethyl-, and trimethyllysine. The latter occurs in calmodulin. Other posttranslational modifications include acetylation. Collagen contains hydroxylysine which is derived from lysine by lysyl hydroxylase. O-Glycosylation of lysine residues in the endoplasmic reticulum or Golgi apparatus is used to mark certain proteins for secretion from the cell.
HO2CCH(NH2)(CH2)4NH2.
H2N-[CH2]4-CH(NH2)-COOH
Behaves similarly to arginine. Contains a long flexible side-chain with a positively-charged end. The flexibility of the chain makes lysine and arginine suitable for binding to molecules with many negative charges on their surfaces. E.g., DNA-binding proteins have their active regions rich with arginine and lysine. The strong charge makes these two amino acids prone to be located on the outer hydrophilic surfaces of the proteins.
Sources
Nutritional sources of
Biosynthesis
As an essential amino acid, lysine is not synthesized in animals, hence it must be ingested as lysine or lysine-containing proteins. In plants and microorganisms, it is synthesized from aspartic acid, which is first converted to β-aspartyl-semialdehyde. Cyclization gives dihydropicolinate, which is reduced to Δ1-piperidine-2,6-dicarboxylate. Ring-opening of this heterocycle gives a series of derivatives of pimelic acid, ultimately affording lysine. Enzymes involves in this biosynthesis include:[1]
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- dihydropicolinate synthase
- Δ1-piperdine-2,6-dicarboxylate dehydrogenase
- N-succinyl-2-amino-6ketopimelate synthase
- succinyl diaminopimelate aminotransferase
- succinyl diaminopimelate desuccinylase
- diaminopimelate epimerase
- diaminopimelate decarboxylase
Metabolism
Lysine is metabolised in mammals to give acetyl-CoA, via an initial transamination with α-ketoglutarate. The bacterial degradation of lysine yields cadaverine by decarboxylation.
Synthesis
Synthetic, racemic lysine has long been known.[2] A practical synthesis starts from caprolactam.[3]
Dietary sources
The human nutritional requirement is 1–1.5 g daily. Used as a dietary supplement. It is the limiting amino acid in all cereal grains, but is plentiful in all pulses (legumes). Fish are also quite rich in lysine. Plants that contain significant amounts of lysine include:[citation needed]
- Buffalo Gourd (10,130–33,000 ppm) in seed
- Berro, Watercress (1,340–26,800 ppm) in herb.
- Soybean (24,290–26,560 ppm) in seed.
- Carob, Locust Bean, St.John's-Bread (26,320 ppm) in seed;
- Common Bean (Black Bean, Dwarf Bean, Field Bean, Flageolet Bean, French Bean, Garden Bean, Green Bean, Haricot, Haricot Bean, Haricot Vert, Kidney Bean, Navy Bean, Pop Bean, Popping Bean, Snap Bean, String Bean, Wax Bean) (2,390–25,700 ppm) in sprout seedling;
- Ben Nut, Benzolive Tree, Jacinto (Sp.), Moringa (aka Drumstick Tree, Horseradish Tree, Ben Oil Tree), West Indian Ben (5,370–25,165 ppm) in shoot.
- Lentil (7,120–23,735 ppm) in sprout seedling.
- Asparagus Pea, Winged Bean (aka Goa Bean) (21,360–23,304 ppm) in seed.
- Fat Hen (3,540–22,550 ppm) in seed.
- Lentil (19,570–22,035 ppm) in seed.
- White Lupin (19,330–21,585 ppm) in seed.
- Black Caraway, Black Cumin, Fennel-Flower, Nutmeg-Flower, Roman Coriander (16,200–20,700 ppm) in seed.
- Spinach (1,740–20,664 ppm).
- Amaranth, Quinoa
Properties
L-Lysine is a necessary building block for all protein in the body. L-Lysine plays a major role in calcium absorption; building muscle protein; recovering from surgery or sports injuries; and the body's production of hormones, enzymes, and antibodies.
Clinical significance
It has been suggested that lysine may be beneficial for those with herpes simplex infections.[4] However, more research is needed to fully substantiate this claim. For more information, refer to Herpes simplex - Lysine.
Lysine can help to alleviate the symptoms of coldsores. They help to speed up the healing process if taken immediately.
ReferencesISBN links support NWE through referral fees
References
- Much of the information in this article has been translated from German Wikipedia.
- Template:RubberBible83rd
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Braun, J. V. “Synthese des inaktiven Lysins aus Piperidin" Berichte der deutschen chemischen Gesellschaft 1909, Volume 42, p 839-846. DOI: 10.1002/cber.190904201134.
- ↑ Eck, J. C.; Marvel, C. S. “dl-Lysine Hydrochlorides” Organic Syntheses, Collected Volume 2, p.374 (1943). http://www.orgsyn.org/orgsyn/pdfs/CV2P0374.pdf
- ↑ Griffith RS, Norins AL, Kagan C. (1978). A multicentered study of lysine therapy in Herpes simplex infection. Dermatologica. 156 (5): 257-267. PMID 640102.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
- Longe, J. L. 2006. The Gale Encyclopedia of Medicine. Detroit: Thomson Gale. ISBN 1414403682.
- Bender, D. A., and A. E. Bender. 2005. A Dictionary of Food and Nutrition. New York: Oxford University Press. ISBN 0198609612.
External links
- Lysine biosynthesis (early stages), Lysine biosynthesis (later stages), and Lysine catabolism at Queen Mary, University of London
- Computational Chemistry Wiki at compchemwiki.org
- L-Lysine at PDRhealth.com
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.