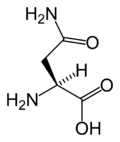
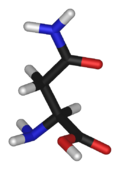
Asparagine
| |
Asparagine | |
| Systematic (IUPAC) name | |
| (2S)-2-amino-3-carbamoyl-propanoic acid | |
| Identifiers | |
| CAS number | 70-47-3 |
| PubChem        | 236 |
| Chemical data | |
| Formula | C4H8N2O3Â |
| Mol. weight | 132.118 |
| SMILES | N[C@@H](CC(N)=O)C(O)=O |
| Complete data | |
Asparagine, also known as asparamide, is α-amino acid that is found in many proteins, particularly in plant proteins, such as in asparagus. Asparagine is closely related to the amino acid aspartic acid, into which it is easily hydrolized.
In humans, the L-isomer of asparagine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. However, asparagine is considered to be a "non-essential amino acid" since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactionsâin this case, synthesized easily from aspartic acid.
The production of asparagine (utilizing various enzymes and chemical compounds), the incorporation of asparagine and other amino acids into proteins in a particular arrangement, and the folding of proteins into a precise three-dimensional configuration involves the coordination of a great number of complex steps, revealing the remarkable harmony in the universe.
Asparagine was first isolated in 1806 from asparagus juice, in which it is abundantâhence its name. Asparagine was the first amino acid to be isolated.
A reaction between asparagine and reducing sugars or reactive carbonyls produces acrylamide (acrylic amide) in food when heated to sufficient temperature, i.e. baking temperatures. Acrylamides are a chemical compound that some consider may pose health risks; they occur primarily in baked goods such as french fries, potato chips, and roasted coffee.
Asparagine's three letter code is ASN, its one letter code is N, and its systematic name is 2-Amino-3-carbamoylpropanoic acid (IUPAC-IUB 1983). A three-letter designation for either asparagine or aspartic acid is Asx (one-letter abbreviation: B).
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acidsâthose amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called αâcarbon (alpha carbon). The general structure of these alpha amino acids is:
R | H2N-C-COOH | H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In asparagine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Asparagine's chemical formula is H2N-CO-CH2-CH(NH2)-COOH, or more generally, C4H8N2O3. It is similar in structure to aspartic acid, but has carboxamide as the side chain's functional group. Essentially, the side chain carboxyl group of aspartic acid is coupled with ammonia, yielding a relatively unreactive neutral amide group. This side chain functional group has importance in asparagine's role in proteins (see function).
Sources
Common dietary sources of asparagine include asparagus, dairy products, potatoes, beef, poultry, meat, and eggs.
Biosynthesis
Asparagine is not an essential amino acid, which means that it can be synthesized in the human body from central metabolic pathway intermediates and is not required in the diet.
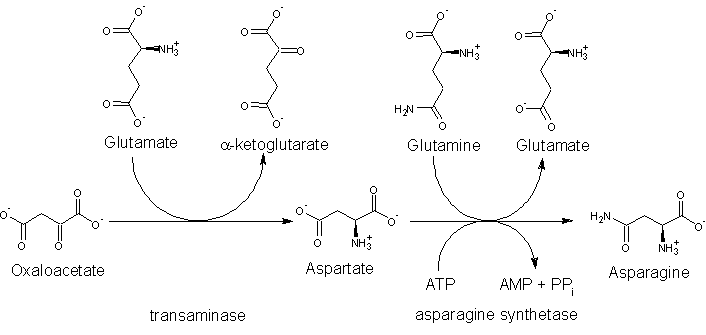
The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate (aspartic acid) using a transaminase enzyme. The enzyme transfers the amino group from glutamate to oxaloacetate producing α-ketoglutarate and aspartate. The enzyme asparagine synthetase then produces asparagine, adenosine monophosphate (AMP), glutamate, and pyrophosphate from aspartate, glutamine, and adenosine triphosphate (ATP). In the asparagine synthetase reaction, ATP is used to activate aspartate, forming ÎČ-aspartyl-AMP. Glutamine donates an ammonium group, which reacts with ÎČ-aspartyl-AMP to form asparagine and free AMP.
Degradation
Asparagine can be degraded easily into aspartate (aspartic acid), which is a glucogenic amino acid. A glucogenic amino acid is an amino acid that can be converted into glucose through gluconeogenesis. Gluconeogenesis is the process of generating glucose from non-sugar carbon substrates like pyruvate, lactate, glycerol, and glucogenic amino acids.
Essentially, L-asparginase hydrolyzes the amide group of asparagine to form aspartate and ammonium. A transaminase converts the aspartate to oxaloacetate, which can then be metabolized in the citric acid cycle or via gluconeogenesis.
The characteristic smell observed in the urine of individuals after their consumption of asparagus is attributed to various metabolic byproducts of asparagine. In 1891, Marceli Nencki claimed that the substance responsible was methanethiol, and Robert White's 1975 research indicated that the substances were various thioesters (Adams 1999). Other likely possibilities include asparagine aminosuccinic monoamide. Allison and McWhirter (1956) indicated that some individuals do not produce this odor after asparagus consumption, and that this is autosomal (on a non-sex chromosome); however, a re-examination in 1980 showed that these individuals are, rather, not able to detect the odor.
Function
Asparagine is necessary for the synthesis of many proteins. Since the asparagine side chain (the carboxamide, see structure) can make efficient hydrogen bond interactions with the peptide backbone, asparagines are often found in proteins near the beginning and end of alpha-helices, and in turn motifs in beta sheets. Its role can be thought as "capping" the hydrogen bond interactions which would otherwise need to be satisfied by the polypeptide backbone. Glutamines have an extra methylene group, have more conformational entropy. and thus are less useful in this regard.
Asparagine also provides key sites for N-linked glycosylationâmodification of the protein chain with the addition of carbohydrate chains.
Asparagine plays an important role in the metabolism of ammonia, which is toxic in the human body. The nervous system needs asparagine to maintain the equilibrium, as well as in amino acid transformation.
ReferencesISBN links support NWE through referral fees
- Adams, C. Why does asparagus make your pee smell funny? Straight Dope, 1999. Retrieved September 12, 2007.
- Allison, A. C., and K. G. McWhirter. âTwo unifactorial characters for which man is polymorphic.â Nature, 178(4536): 748-749, 1956.
- Doolittle, R. F. âRedundancies in protein sequences.â In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press, 1989. ISBN 0306431319
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB, 1983. Retrieved September 12, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing, 2000. ISBN 1572591536
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.