Endocytosis
Endocytosis is a process where cells ingest material (macromolecules, low-molecular weight molecules, and particles) from outside the cell by enclosing it with a portion of their cell membrane and bringing it into the cell in a membrane-bound vesicle. In other words, a small portion of the cell membrane (plasma membrane) invaginates and progressively encloses the extracellular substance, and then pinches off inside the cell to form a membrane-bound, intracellular vesicle with the ingested material (Alberts et al. 1989). The function of endocytosis is the opposite of exocytosis, in which materials packaged in secretory vesicles inside the cell fuse with the plasma membrane and open to the exterior space, releasing the material (Alberts et al. 1989).
Endocytosis is used by cells because most substances important to them are large polar molecules, and thus cannot pass through the hydrophobic plasma membrane. The material tends to remain sequestered in vesicles and does not mix with other macromolecules or organelles in the cytoplasm, other than specific membranes for which it is destined, causing a directed transfer between the inside and outside of the cell (Alberts et al. 1989). Typically, the internalized molecules trapped in the intracellular vesicles eventually fuse with the membrane-bound lysosomes and are degraded (Khalil et al. 2006).
Endocytosis may involve the ingestion of fluid and solutes or the ingestion of large particles (such as microorganisms and cell debris) (Alberts et al. 1989). The mechanism of internalizing macromolecules into cells is an intricately coordinated process. Through understanding the various endocytic uptake pathways, mechanisms can be improved for successful non-viral delivery of therapeutic genes to the particular target cells, such as necessary for gene therapy (Khalil et al. 2006).
Overview and categories of endocytosis
Many substances needed by the cell cannot cross the plasma membrane because of their size or hydrophilic nature. Endocytosis, or the vesicular uptake of extracellular materials, is a means whereby the macromolecules can be internalized by a cell. Khalil et al. (2006) succinctly define endocytosis as "the cellular uptake of macromolecules and solutes into membrane-bound vesiciles derived by the invagination and pinching off of pieces of the plasma membrane."
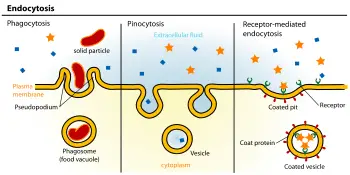
The absorption of material from the outside environment of the cell is commonly divided into two broad categories: phagocytosis and pinocytosis. These two categories can be distinguished primarily by the size of the material ingested and vesicles formed and by phagocytosis being dependent on actin polymerization as a key step in the ingestion (Liu 2003).
Phagocytosis. Phagocytosis (literally, "cell-eating") is the process by which cells ingest large objects, such as cells that have undergone apoptosis, bacteria, or viruses. The membrane folds around the object, and the object is sealed off into large vesicles, called phagosome or vacuoles, generally larger than 250 nm in diameter (Alberts et al. 1989).
Pinocytosis. Pinocytosis (literally, cell-drinking). This process involves the uptake of fluids and solutes using small vesicles, generally less than 150 nm in diameter (Alberts et al. 1989).
Many of the molecules ingested by cells by either phagocytosis or pinocytosis end up in lysosomes to be degraded. Phagosomes fuse with lysosomes to form phagolysosomes, whereas fluid and macromolecules ingested by pinocytosis first are transfered to endosomes (membrane-bound organelles) and then carried to lysosomes (Alberts et al. 1989). Amino acids, sugars, and nucleotides that occur as breakdown products from material from phagosomes and endosomes fusing with lysosomes is transported across the lysosomal membrane into the cytosol, where they can be used by cell (Alberts et al. 1989).
Phagocytosis generally is restricted to specialized phagocytic mammalian cells, such as macrophages, monocytes, and neutrohiles, which help to clear large pathogens such as bacteria or yeast, or debris such as dead cells and fat deposits in arteries (Khalil et al. 2006).
Pinocytosis, on the other hand, is found in all cells (Khalil et al. 2006; Alberts et al. 1989). For this reason, the terms pinocytosis and endocytosis occasionally are considered to be synonymous (Khalil et al. 2006).
Modes of endocytosis
Three modes of endocytosis can be delineated kinetically: fluid-phase, adsorptive, and receptor-mediated endocytosis (Khalil et al. 2006).
Fluid-phase endocytosis. Fluid-phase endocytosis is a low efficiency, nonspecific process that involves the bulk uptake of solutes in exact proportion to their concentration in the extracellular fluid (Khalil et al. 2006).
Absorptive. Absorptive endocytosis molecules are bound to the cell surface and concentrated before internalization, with the molecules interacting preferentially with generic complementary binding sites, such as lectin or charged interaction (Khalil et al. 2006).
Receptor-mediated endocytosis. Receptor-mediated endocytosis also involves concentration of the molecules, with certain ligands binding to receptors on the cell surface and becoming concentrated before internalization. The cytoplasm membrane folds inward to form coated pits, such as clathrin-coated pits. These inward budding vesicles bud to form cytoplasmic vesicles.
Endocytic uptake pathways (specifically pinocytic pathways)
There are at least four morphologically distinct pinocytic pathways: macropinocytosis, caveolar endocytosis, clathrin-mediated endocytosis, and clathrin/caveole-independent endocystosis. These four pathways differ in composition of the coat (if any), the size of the membrane-bound, intracellular vesicles, and the fate of the internalized material (Khalil et al. 2006). A fifth endocytic uptake pathway is phagocytosis, which is described above.
Macropinocytosis. Macropinocytosis is the invagination of the cell membrane to form a large endocytoic vesicles of irregular shape and size, generated by actin-driven envagination of the plasma membrane, with the intracellular vesicle filled with extracellular fluid (and molecules within it) (Khalil et al. 2006). The filling of the pocket occurs in a non-specific manner. The vesicle then travels into the cytosol and fuses with other vesicles such as endosomes and lysosomes. Macropinosomes are large vesicles that can have a diameter of up to 2.5 µm, in contrast to the smaller pinosomes and endocytic (endocytotic) vacuoles that have diameters typcially of 0.1 to 0.9 µm (Pellegrin et al. 2002).
Clathrin-mediated endocytosis. Clathrin-mediated endocytosis is the specific uptake of large extracellular molecules, such as proteins, membrane localized receptors, and ion-channels. These receptors are associated with the cytosolic protein clathrin, which initiates the formation of a vesicle by forming a crystalline coat on the inner surface of the cell's membrane. Clathrin-mediated endocytosis is the major endocytoic pathway, found in all mammalian cells, and carries out the continuous uptake of essential nutrients, growth factors, antigens, and pathogens, including the cholesterol-laden, low-density lipoprotein (LDL) (Khalil et al. 2006).
Caveolae-mediated endocytosis. Caveolae are small hydrophobic membrane areas that consist of the protein caveolin-1 with a bilayer enriched in cholesterol and glycolipids. Caveolae typically are described as flask-shape pits in the plasma membrane that resemble the shape of a cave (hence the name caveolae), however they can also be flat, tubular, or detached vesicles (Khalil et al. 2006). Uptake of extracellular molecules is also believed to be specifically mediated via receptors in caveolae. Caveolae are found in many cell types and especially in endothelial cells (Khalil et al. 2006). Potocytosis is a term used for the sequestration and transport of small molecules by caveolae without the merging of an endocytic (or endocytotic) vesicle with endosomes (Khalil et al. 2006; Liu 2003). For example, the uptake of folic acid is believed to involve binding to folate receptors in invaginated caveole, but the caveolae stay attached and the ligand is released from the receptors and 5-methyltetrahydrofolic acid moves acrose the caveolar membrane into the cytosol (Khalil et al. 2006). Becasue caveolar update is believed not to lead to lysosomal degradation, it seems advantageous in terms of DNA delivery for gene delivery strategies (Khalil et al. 2006).
Clathrin/caveolae independent endocytosis. Although most receptors in receptor-mediated endocytosis are internalized by clathrin-coated pits, there are other pinocytic pathways that have been suggested other than clathrin-mediated endocytosis or involving caveole (Khalil et al. 2006).
Clathrin-mediated endocytosis
The major and best understood route for endocytosis in most cells is that mediated by the molecule clathrin. This large protein assists in the formation of a coated pit on the inner surface of the plasma membrane of the cell. This pit then buds into the cell to form a coated vesicle in the cytoplasm of the cell. In so doing, it brings into the cell not only a small area of the surface of the cell but also a small volume of fluid from outside the cell.
Vesicles selectively concentrate and exclude certain proteins during formation and are not representative of the membrane as a whole. AP2 adaptors are multi-subunit complexes that perform this function at the plasma membrane. The best-understood receptors that are found concentrated in coated vesicles of mammalian cells are the LDL receptor (which removes LDL from circulating blood), the transferrin receptor (which brings ferric ions bound by transferrin into the cell), and certain hormone receptors (such as that for EGF).
At any one moment, about 25 percent of the plasma membrane of a fibroblast is made up of coated pits. As a coated pit has a life of about a minute before it buds into the cell, a fibroblast takes up its surface by this route about once every 50 minutes. Coated vesicles formed from the plasma membrane have a diameter of about 100 nm and a lifetime measured in a few seconds. Once the coat has been shed, the remaining vesicle fuses with endosomes and proceeds down the endocytic pathway. The actual budding-in process, whereby a pit is converted to a vesicle, is carried out by clathrin assisted by a set of cytoplasmic proteins, which includes dynamin and adaptors such as adaptin.
Coated pits and vesicles were first seen in thin sections of tissue in the electron microscope by Thomas Roth and Keith Porter in 1964. The importance of them for the clearance of LDL from blood was discovered by R. G Anderson, Michael S. Brown and Joseph L. Goldstein in 1976. Coated vesicles were first purified by Barbara Pearse, who discovered the clathrin coat molecule, also in 1976.
ReferencesISBN links support NWE through referral fees
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. Molecular Biology of the Cell, 2nd edition. New York: Garland Publishing, 1989. ISBN 0824036956.
- Khalil, I. A., K. Kogure, H. Akita, and H. Harashima. 2006. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery Pharmacological Reviews 58(1); 32-45. Retrieved August 8, 2008.
- Liu, J. 2003. Endocytosis and signal transduction: Basic science update Biological Research for Nursing 5(2): 117-128. Retrieved August 8, 2008.
- Pellegrin, P., A. Fernandez, N. J. C. Lamb, and R. Bennes. 2002. Macromolecular uptake is a spontaneous event during mitosis in culture fibroblasts: Implications for vector-dependent plasmid transfection Molecular Biology of the Cell 13(2): 570-578. Retrieved August 8, 2008.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.