Difference between revisions of "Allotropy" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
|||
| Line 1: | Line 1: | ||
| − | [[Image:Diamond and graphite.jpg|thumb|300px|Diamond and graphite are two allotropes of | + | {{Claimed}} |
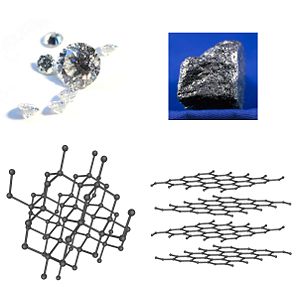
| + | [[Image:Diamond and graphite.jpg|thumb|300px|Diamond and graphite are two allotropes of carbon—that is, they are pure forms of the same element that differ in structure.]] | ||
| + | |||
| + | <<Contrast allotropy with polymorphism>> | ||
| + | |||
| + | '''Allotropy''' (Gr. ''allos'', other, and ''tropos'', manner) is a behavior exhibited by certain [[chemical elements]]: these elements can exist in two or more different forms, known as ''allotropes'' of that element. In each different allotrope, the element's atoms are bonded together in a different manner. | ||
For example, the element [[carbon]] has two common allotropes: [[diamond]], where the carbon atoms are bonded together in a [[tetrahedral]] lattice arrangement, and [[graphite]], where the carbon atoms are bonded together in sheets of a hexagonal lattice. | For example, the element [[carbon]] has two common allotropes: [[diamond]], where the carbon atoms are bonded together in a [[tetrahedral]] lattice arrangement, and [[graphite]], where the carbon atoms are bonded together in sheets of a hexagonal lattice. | ||
| Line 22: | Line 27: | ||
* [[diamond]] - an extremely hard, transparent crystal, with the carbon atoms arranged in a tetrahedral lattice. A poor conductor. | * [[diamond]] - an extremely hard, transparent crystal, with the carbon atoms arranged in a tetrahedral lattice. A poor conductor. | ||
* [[graphite]] - a soft, black, flaky solid, a moderate electrical conductor. The C atoms are bonded in flat hexagonal lattices, which are then layered in sheets. | * [[graphite]] - a soft, black, flaky solid, a moderate electrical conductor. The C atoms are bonded in flat hexagonal lattices, which are then layered in sheets. | ||
| − | * [[fullerene]] - (including the "buckyball" | + | * [[fullerene]] - (including the "buckyball," C<sub>60</sub>) |
[[Phosphorus]]: | [[Phosphorus]]: | ||
| Line 30: | Line 35: | ||
[[Oxygen]]: | [[Oxygen]]: | ||
| − | * [[dioxygen]], O<sub>2</sub> - | + | * [[dioxygen]], O<sub>2</sub> - colorless |
*[[ozone]], O<sub>3</sub> - blue | *[[ozone]], O<sub>3</sub> - blue | ||
*[[tetraoxygen]], O<sub>4</sub> - red | *[[tetraoxygen]], O<sub>4</sub> - red | ||
| Line 40: | Line 45: | ||
*Molecular sulfur - sulphur tends to form ring molecules such as S<sub>7</sub> and S<sub>12</sub> | *Molecular sulfur - sulphur tends to form ring molecules such as S<sub>7</sub> and S<sub>12</sub> | ||
| − | [[Plutonium]] has six distinct solid allotropes under 'normal' pressures. Their densities vary within a ratio of some 4:3, which vastly complicates all kinds of work with the metal (particularly casting, machining, and storage). | + | [[Plutonium]] has six distinct solid allotropes under 'normal' pressures. Their densities vary within a ratio of some 4:3, which vastly complicates all kinds of work with the metal (particularly casting, machining, and storage). A seventh plutonium allotrope exists at very high pressures, which adds further difficulties in exotic applications. |
| + | |||
| + | == See also == | ||
| + | |||
| + | * [[Carbon]] | ||
| + | * [[Diamond]] | ||
| + | * [[Graphite]] | ||
| + | * [[Oxygen]] | ||
| + | * [[Phosphorus]] | ||
| + | * [[Plutonium]] | ||
| + | * [[Polymorphism (materials science)]] | ||
| + | * [[Pyrite]] | ||
| + | * [[Sulfur]] | ||
| + | |||
| + | == References == | ||
| + | <<Needs 3 refs>> | ||
==External links== | ==External links== | ||
| − | *Allotrope in ''IUPAC Compendium of Chemical Terminology'', Electronic version, | + | * [http://goldbook.iupac.org/A00243.html Allotrope] in ''IUPAC Compendium of Chemical Terminology'', Electronic version. Retrieved May 8, 2007. |
| + | [[Category:Physical sciences]] | ||
| + | [[Category:Chemistry]] | ||
[[Category:Inorganic chemistry]] | [[Category:Inorganic chemistry]] | ||
| − | + | {{credit|128517287}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 13:23, 8 May 2007
<<Contrast allotropy with polymorphism>>
Allotropy (Gr. allos, other, and tropos, manner) is a behavior exhibited by certain chemical elements: these elements can exist in two or more different forms, known as allotropes of that element. In each different allotrope, the element's atoms are bonded together in a different manner.
For example, the element carbon has two common allotropes: diamond, where the carbon atoms are bonded together in a tetrahedral lattice arrangement, and graphite, where the carbon atoms are bonded together in sheets of a hexagonal lattice.
Note that allotropy refers only to different forms of an element within the same phase or state of matter (i.e. different solid, liquid or gas forms) - the changes of state between solid, liquid and gas in themselves are not considered allotropy. For some elements, allotropes can persist in different phases - for example, the two allotropes of oxygen (dioxygen and ozone), can both exist in the solid, liquid and gaseous states. Conversely, some elements do not maintain distinct allotropes in different phases: for example phosphorus has numerous solid allotropes, which all revert to the same P4 form when melted to the liquid state.
The concept of allotropy was originally devised by the Swedish scientist Baron Jons Jakob Berzelius (1779-1848).
Differences in properties of an element's allotropes
Allotropes of the same element can typically exhibit quite different physical properties and chemical behaviour, even though they contain nothing else but atoms of that element. They may have different colours, odour, hardnesses, electrical and thermal conductivies, etc.
The change between different allotropic forms of an element is often triggered by pressure and temperature, and many allotropes are only stable in the correct conditions. For instance, iron only changes from ferrite to austenite above 723°C, and tin undergoes a process known as tin pest at 13.2°C and below.
Examples of allotropes
Typically, elements capable of variable coordination number and/or oxidation states tend to exhibit greater numbers of allotropic forms. Another contributing factor is the ability of an element to catenate. Allotropes are typically more noticeable in non-metals and metalloids.
Examples of allotropes include:
- diamond - an extremely hard, transparent crystal, with the carbon atoms arranged in a tetrahedral lattice. A poor conductor.
- graphite - a soft, black, flaky solid, a moderate electrical conductor. The C atoms are bonded in flat hexagonal lattices, which are then layered in sheets.
- fullerene - (including the "buckyball," C60)
- Red Phosphorus - polymeric solid
- White Phosphorus - crystalline solid
- Black Phosphorus - semiconductor, analogous to graphite
- dioxygen, O2 - colorless
- ozone, O3 - blue
- tetraoxygen, O4 - red
- Plastic (amorphous) sulfur - polymeric solid
- Rhombic sulfur - large crystals composed of S8 molecules
- Monoclinic sulfur - fine needle-like crystals
- Molecular sulfur - sulphur tends to form ring molecules such as S7 and S12
Plutonium has six distinct solid allotropes under 'normal' pressures. Their densities vary within a ratio of some 4:3, which vastly complicates all kinds of work with the metal (particularly casting, machining, and storage). A seventh plutonium allotrope exists at very high pressures, which adds further difficulties in exotic applications.
See also
ReferencesISBN links support NWE through referral fees
<<Needs 3 refs>>
External links
- Allotrope in IUPAC Compendium of Chemical Terminology, Electronic version. Retrieved May 8, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.