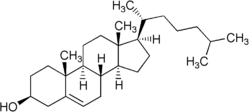
Steroid
A steroid is any of a group of naturally or synthetic, fat-soluable, organic compounds belonging to the class of lipids and characterized by a molecular ring structure involving four fused rings totally 17 carbon atoms: three fused six-carbon rings and one five-carbon ring. The three-dimensional configuration, type of additional side chains and rings determines the type of steroid.
Steroids are ubiquitous in nature, being found in animals, plants, fungi, and protozoa, and perform many biological functions, including involvement in cell membranes and serving as hormones. Steorids include the sterols, bile acids, adrenal and sex hormones. Cholesterol is the most abundant steorid in mammalian cells.
Chemistry
Along with proteins, nucleic acids, and carbohydrates, lipids are one of the major classes of biologically important molecules (or biomolecules). They are water-insoluble, organic compounds that are highly soluble in nonpolar organic solvents. subgroup of lipids called triglycerides. Unlike other groups of molecules, lipids comprise a broad and diverse range of structures, which also include phospholipids (components of cell membranes), sterols (most notably cholesterol, and the steroid hormones), and more complex lipid derivatives such as glycolipids (sugar-linked lipids).
is a terpenoid lipid characterized by a carbon skeleton with four fused rings. Different steroids vary in the functional groups attached to these rings. All steroids are derived either from the sterol lanosterol (animals and fungi) or the sterol cycloartenol (plants). Both sterols are derived from the cyclization of the triterpene squalene.[1]
Sterol lipids: structure and signaling
Cholesterol
Cholesterol is a sterol lipid (a combination steroid and alcohol) with the chemical formula C27H45OH. It is found in the cell membranes of all human body tissues, and transported in the blood plasma of all animals. Lesser amounts of cholesterol are also found in plant membranes.
Cholesterol is an important component of cell membranes, which enhances their fluidity. Cholesterol also aids in the manufacture of bile (which helps digest fats), and is also important for the metabolism of fat-soluble vitamins.
Cholesterol and triglycerides are transported in body fluids in the form of lipoproteins, the natural carrier molecules of the body, which are classified according to density. When doctors talk to their patients about the health concerns of cholesterol, they are often referring to "bad cholesterol," or low-density lipoprotein (LDL). "Good cholesterol" is high-density lipoprotein (HDL). Both types of cholesterol have biologically important roles in animals: LDL transports cholesterol to peripheral tissues and regulates the synthesis of cholesterol at these sites, while HDL "sweeps" the blood of cholesterol released into the plasma from dying cells and from membranes undergoing turnover (regeneration). However, high levels of LDL in the blood may lead to the build-up of atherosclerotic plaques in arteries, which may in turn result in cardiovascular disease.
Steroid hormones
Cholesterol is an important precursor of the steroid hormones. Steroid hormones produce their physiological effects by binding to steroid hormone receptor proteins, which causes changes in gene transcription and cell function.
The five major classes of steroids are as follows:
- Androgens (such as testosterone) are responsible for the development of male secondary sex characteristics.
- Glucocorticoids enable animals to respond to stress. They regulate many aspects of metabolism and immune function, and are often prescribed by doctors to reduce inflammatory conditions like asthma and arthritis.
- Mineralocorticoids help maintain blood volume and control renal excretion of electrolytes.
- Estrogens and progestagens are two classes of sex steroids, a subset of the hormones that produce sex differences or support reproduction.
Origin
Steroids include estrogen (U.S spelling) or oestrogen (UK spelling), progesterone and androgen. Oestrogen and progesterone are made primarily in the ovary and in the placenta during pregnancy and testosterone in the testes. Certain neurons and glia in the central nervous system (CNS) express the enzymes that are required for the local synthesis of pregnane neurosteroids, either de novo or from peripherally derived sources.
Classification
Taxonomical/Functional
Some of the common categories of steroids:
- Animal steroids
- Insect steroids
- Ecdysteroids such as ecdysterone
- Vertebrate steroids
- Sex steroids are a subset of sex hormones that produce sex differences or support reproduction. They include androgens, estrogens, and progestagens.
- Corticosteroids include glucocorticoids and mineralocorticoids. Glucocorticoids regulate many aspects of metabolism and immune function, whereas mineralocorticoids help maintain blood volume and control renal excretion of electrolytes.
- Anabolic steroids are a class of steroids that interact with androgen receptors to increase muscle and bone synthesis. There are natural and synthetic anabolic steroids. These are the steroids used by athletes to increase performance.
- Cholesterol which modulates the fluidity of cell membranes and is the principle constituent of the plaques implicated in atherosclerosis.
- Insect steroids
- Plant steroids
- Phytosterols
- Brassinosteroids
- Fungus steroids
- Ergosterols
Structural
It is also possible to classify steroids based upon their chemical composition. One example of how MeSH performs this classification is available at Wikipedia:MeSH D04#MeSH D04.808 --- steroids.
Steroid hormones
Steroid hormones are steroids which act as hormones. Mammalian steroid hormones can be grouped into five groups by the receptors to which they bind: glucocorticoids, mineralocorticoids, androgens, estrogens, and progestagens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors, though technically sterols rather than steroids.
Overview
The natural steroid hormones are generally synthesized from cholesterol in the gonads and adrenal glands. These forms of hormones are lipids. They can enter the cell membrane quite easily and enter right into the nuclei. Steroid hormones are generally carried in the blood bound to specific carrier proteins such as sex hormone binding globulin or corticosteroid binding globulin. Further conversions and catabolism occurs in the liver, other "peripheral" tissues, and in the target tissues.
Because steroids and sterols are lipid soluble, they can diffuse fairly freely from the blood through the cell membrane and into the cytoplasm of target cells. In the cytoplasm the steroid may or may not undergo an enzyme-mediated alteration such as reduction, hydroxylation, or aromatization. In the cytoplasm, the steroid binds to the specific receptor, a large metalloprotein. Upon steroid binding, many kinds of steroid receptor dimerize: two receptor subunits join together to form one functional DNA-binding unit that can enter the cell nucleus. In some of the hormone systems known, the receptor is associated with a heat shock protein which is released on the binding of the ligand, the hormone. Once in the nucleus, the steroid-receptor ligand complex binds to specific DNA sequences and induces transcription of its target genes.
Anabolic steroids
External links
- Michael W. King's Medical Biochemistry. Steroids and retinoids are both terpenes which are hydrophobic, pass through cell membranes and bind to intracellular receptors. However, retinoic acid is not a steroid because is does not have the defining ring structure. See: Steroids and Related Hydrophobic Molecules.
- "Biochemistry" by Jeremy M. Berg, John L. Tymoczko and Lubert Stryer (2002) W. H. Freeman and Co. steroid topics in this
ReferencesISBN links support NWE through referral fees
- Agis-Balboa RC. et al. (2006). "Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis". Proc Natl Acad Sci U S A 103, 14602-14607. PMID 16984997
- Belelli D. and Lambert JJ. (2005). "Neurosteroids: endogenous regulators of the GABAA receptor". Nature Reviews Neuroscience 6, 565-575. PMID 15959466. Review
- Pinna G. et al. (2005). "Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake". Psychopharmacology (Berl) 186, 362-372. PMID 16432684. Review
- Dubrovsky BO. (2005). "Steroids, neuroactive steroids and neurosteroids in psychopathology". Prog Neuropsychopharmacol Biol Psychiatry 29, 169-192, PMID 15694225. Review
- Mellon SH. and Griffin LD. (2002) "Neurosteroids: biochemistry and clinical significance". Trends Endocrinol Metab 13, 35-43. PMID 11750861. Review
| |||||||||||||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.