Difference between revisions of "Steroid" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 1: | Line 1: | ||
{{Contracted}} | {{Contracted}} | ||
| − | [[Image: | + | [[Image:Steroid-nomenclature.png|thumb|right|250px|Steroids, such as cholesterol and the steroid hormones, are characterized by a carbon skeleton with four fused rings. They are distinguished by the functional groups attached to the rings.]] |
| − | + | A '''steroid''' is any of a group of naturally or synthetic, fat-soluble, organic compounds belonging to the class of [[lipid]]s and characterized by a molecular core of four fused rings totaling 17 carbon atoms: three fused six-carbon rings and one five-carbon ring. The type of steroid is determined by the three-dimensional configuration and the type of additional side chains and rings. | |
| − | A '''steroid''' is any of a group of naturally or synthetic, fat- | ||
| − | |||
| − | |||
| − | |||
| + | Steroids are ubiquitous in nature, being found in animals, plants, fungi, and protozoa, and they perform many biological functions, including involvement in cell membranes and serving as hormones. Steorids include the sterols, bile acids, adrenal and sex hormones. Cholesterol is the most abundant steorid in mammalian cells. | ||
==Chemistry== | ==Chemistry== | ||
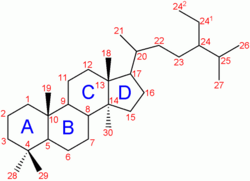
| + | [[Image:Lanosterol structure.svg|thumb|right|250px|Steroid skeleton of [[lanosterol]]. The total number of carbons (30) reflects its [[triterpenoid]] origin. In some steroids some carbons may be removed (such as carbon 18) or added (such as carbons 24<sup>1</sup> and 24<sup>2</sup>) in downstream biosynthetic reactions.]] | ||
| + | Steroids belong to the class of biochemicals called lipids. Along with [[protein]]s, [[nucleic acid]]s, and [[carbohydrate]]s, lipids are one of the major classes of biologically important molecules (or biomolecules). They are water-insoluble, organic compounds that are highly soluble in nonpolar organic solvents. In addition to steroids, other lipids include [[fatty acid]]s, glycerides (such as [[fat|triglyceride]]s or fat), [[phospholipid]]s, and more complex lipid derivatives such as glycolipids (sugar-linked lipids). | ||
| − | + | A steroid is a [[lipid]] characterized by a [[carbon]] skeleton with four fused rings known as a cyclopentanoperhydrophenanthrene ring system. There are three fused six-carbon rings (cyclohexane) and a five-carbon ring (cyclopentane), fused such that there are a total of 17 carbon attoms in the molecular nucleus. Different steroids vary in the functional groups attached to these rings. All steroids are derived either from the [[sterol]] [[lanosterol]] (animals and fungi) or the sterol [[cycloartenol]] (plants). Both sterols are derived from the cyclization of the triterpene squalene (IUBMB 2000). | |
| − | |||
| − | + | ==Classification== | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
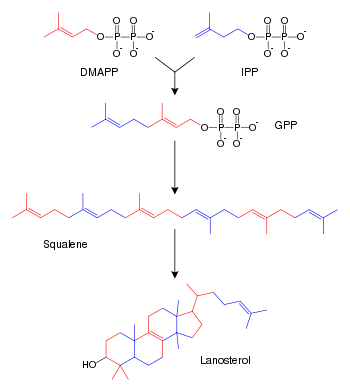
[[Image:Sterol synthesis.svg|thumb|right|350px|Simplified version of the steroid synthesis pathway with the intermediates [[isopentenyl pyrophosphate]] (IPP), [[dimethylallyl pyrophosphate]] (DMAPP), [[geranyl pyrophosphate]] (GPP) and [[squalene]] shown. Some intermediates are omitted.]] | [[Image:Sterol synthesis.svg|thumb|right|350px|Simplified version of the steroid synthesis pathway with the intermediates [[isopentenyl pyrophosphate]] (IPP), [[dimethylallyl pyrophosphate]] (DMAPP), [[geranyl pyrophosphate]] (GPP) and [[squalene]] shown. Some intermediates are omitted.]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
Some of the common categories of steroids: | Some of the common categories of steroids: | ||
*Animal steroids | *Animal steroids | ||
| Line 65: | Line 29: | ||
**[[Ergosterol]]s | **[[Ergosterol]]s | ||
| − | == | + | |
| − | It is also | + | |
| + | ==Cholesterol== | ||
| + | [[Image:Cholesterol structure.png|thumb|right|249px|The chemical structure of cholesterol, showing the molecular core of 17 carbon atoms in four fused rings.]] | ||
| + | '''Cholesterol''' is a [[sterol]] lipid (a combination [[steroid]] and [[alcohol]]) with the chemical formula C<sub>27</sub>H<sub>45</sub>OH. It is found in the cell membranes of all [[human body]] tissues, and transported in the [[blood]] plasma of all [[animal]]s. Lesser amounts of cholesterol are also found in [[plant]] membranes. | ||
| + | |||
| + | Cholesterol is an important component of cell membranes, which enhances their fluidity. Cholesterol also aids in the manufacture of [[bile]] (which helps digest fats), and is also important for the metabolism of fat-soluble [[vitamin]]s. | ||
==Steroid hormones== | ==Steroid hormones== | ||
| − | '''Steroid hormones''' are [[steroid]]s which act as [[hormone]]s. Mammalian steroid hormones can be grouped into five groups by the [[receptor (biochemistry)|receptor]]s to which they bind: [[glucocorticoid]]s, [[mineralocorticoid]]s, [[androgen]]s, [[estrogen]]s, and [[progestagen]]s. [[Vitamin D]] derivatives are a sixth closely related hormone system with homologous receptors, though technically [[sterol]]s rather than steroids. | + | [[Cholesterol]] is an important precursor of the ''steroid hormones''. '''Steroid hormones''' are [[steroid]]s which act as [[hormone]]s. Mammalian steroid hormones can be grouped into five groups by the [[receptor (biochemistry)|receptor]]s to which they bind: [[glucocorticoid]]s, [[mineralocorticoid]]s, [[androgen]]s, [[estrogen]]s, and [[progestagen]]s. [[Vitamin D]] derivatives are a sixth closely related hormone system with homologous receptors, though technically [[sterol]]s rather than steroids. |
| + | Steroid hormones produce their physiological effects by binding to steroid hormone receptor proteins, which causes changes in [[gene]] transcription and cell function. These hormones include: | ||
| + | *[[Androgens]] (such as [[testosterone]]) are responsible for the development of male secondary sex characteristics. | ||
| + | *Glucocorticoids enable animals to respond to [[Stress (medical)|stress]]. They regulate many aspects of [[metabolism]] and [[immune system|immune function]], and are often prescribed by doctors to reduce [[inflammation|inflammatory conditions]] like [[asthma]] and [[arthritis]]. | ||
| + | *Mineralocorticoids help maintain blood volume and control [[kidney|renal]] excretion of electrolytes. | ||
| + | *[[Estrogen]]s and progestagens are two classes of sex steroids, a subset of the hormones that produce sex differences or support [[reproduction]]. | ||
| − | |||
The natural steroid hormones are generally synthesized from [[cholesterol]] in the [[gonad]]s and [[adrenal gland]]s. These forms of hormones are lipids. They can enter the cell membrane quite easily and enter right into the nuclei. Steroid hormones are generally carried in the blood bound to specific carrier [[protein]]s such as sex hormone binding globulin or corticosteroid binding globulin. Further conversions and catabolism occurs in the liver, other "peripheral" tissues, and in the target tissues. | The natural steroid hormones are generally synthesized from [[cholesterol]] in the [[gonad]]s and [[adrenal gland]]s. These forms of hormones are lipids. They can enter the cell membrane quite easily and enter right into the nuclei. Steroid hormones are generally carried in the blood bound to specific carrier [[protein]]s such as sex hormone binding globulin or corticosteroid binding globulin. Further conversions and catabolism occurs in the liver, other "peripheral" tissues, and in the target tissues. | ||
Because steroids and sterols are [[lipid]] soluble, they can [[diffusion|diffuse]] fairly freely from the blood through the [[cell membrane]] and into the [[cytoplasm]] of target cells. In the cytoplasm the steroid may or may not undergo an [[enzyme]]-mediated alteration such as reduction, hydroxylation, or aromatization. In the cytoplasm, the steroid binds to the specific receptor, a large metalloprotein. Upon steroid binding, many kinds of steroid receptor [[dimer|dimerize]]: two receptor subunits join together to form one functional [[DNA]]-binding unit that can enter the [[cell nucleus]]. In some of the hormone systems known, the receptor is associated with a [[heat shock protein]] which is released on the binding of the [[ligand]], the hormone. Once in the nucleus, the steroid-receptor ligand complex binds to specific [[DNA]] sequences and induces transcription of its target [[gene]]s. | Because steroids and sterols are [[lipid]] soluble, they can [[diffusion|diffuse]] fairly freely from the blood through the [[cell membrane]] and into the [[cytoplasm]] of target cells. In the cytoplasm the steroid may or may not undergo an [[enzyme]]-mediated alteration such as reduction, hydroxylation, or aromatization. In the cytoplasm, the steroid binds to the specific receptor, a large metalloprotein. Upon steroid binding, many kinds of steroid receptor [[dimer|dimerize]]: two receptor subunits join together to form one functional [[DNA]]-binding unit that can enter the [[cell nucleus]]. In some of the hormone systems known, the receptor is associated with a [[heat shock protein]] which is released on the binding of the [[ligand]], the hormone. Once in the nucleus, the steroid-receptor ligand complex binds to specific [[DNA]] sequences and induces transcription of its target [[gene]]s. | ||
| + | |||
| + | |||
| + | ==Origin== | ||
| + | Steroids include [[estrogen]] (U.S spelling) or oestrogen (UK spelling), [[progesterone]] and [[androgen]]. Oestrogen and progesterone are made primarily in the [[ovary]] and in the [[placenta]] during pregnancy and [[testosterone]] in the [[testes]]. Certain [[neurons]] and [[glia]] in the [[central nervous system]] (CNS) express the [[enzymes]] that are required for the local synthesis of [[pregnane]] [[neurosteroids]], either [[de novo]] or from peripherally derived sources. | ||
==Anabolic steroids== | ==Anabolic steroids== | ||
| + | |||
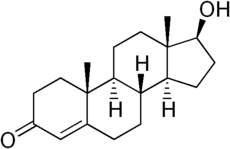
| + | [[Image:Testosterone structure.png|thumb|230px|Chemical structure of the natural anabolic hormone [[testosterone]], 17b-hydroxy-4-androsten-3-one.]] | ||
| + | '''Anabolic androgenic steroids''' or AAS are a class of natural and synthetic [[steroid]] [[hormone]]s that promote cell growth and division, resulting in growth of several types of tissues, especially muscle and bone. Different anabolic androgenic steroids have varying combinations of [[androgen]]ic ''and'' [[anabolism|anabolic]] properties, and are often referred to in medical texts as AAS (anabolic/androgenic steroids). [[Anabolism]] is the metabolic process that builds larger molecules from smaller ones. | ||
| + | |||
| + | Anabolic steroids were first discovered in the early 1930s and have since been used for numerous medical purposes including stimulation of bone growth, [[appetite]], [[puberty]], and [[muscle growth]]. The most widespread use of anabolic steroids is their use for chronic wasting conditions, such as [[cancer]] and [[AIDS]]. Anabolic steroids can produce numerous physiological effects including increases in protein synthesis, muscle mass, strength, appetite and bone growth. Anabolic steroids have also been associated with numerous side effects when administered in excessive doses and these include elevated [[cholesterol]] (increase in [[Low density lipoprotein|LDL]], decreased [[High density lipoprotein|HDL]] levels), [[Acne vulgaris|acne]], elevated [[blood pressure]], [[hepatotoxicity]], and alterations in [[left ventricle]] [[Morphology (biology)|morphology]]. | ||
| + | |||
| + | Today anabolic steroids are controversial because of their widespread use in competitive [[sports]] and their associated side effects. While there are numerous health issues associated with excessive anabolic steroid use, public understanding of the true risks remains limited. Anabolic steroids are controlled in a few countries including the United States, where they are listed as Schedule III in the [[Controlled Substances Act]], as well as Canada and Britain who also have laws controlling their use and distribution. | ||
| + | |||
| + | |||
== External links == | == External links == | ||
| Line 94: | Line 80: | ||
*[[Mellon SH. and Griffin LD.]] (2002) "Neurosteroids: biochemistry and clinical significance". ''Trends Endocrinol Metab'' '''13''', 35-43. PMID 11750861. ''Review'' | *[[Mellon SH. and Griffin LD.]] (2002) "Neurosteroids: biochemistry and clinical significance". ''Trends Endocrinol Metab'' '''13''', 35-43. PMID 11750861. ''Review'' | ||
| + | |||
| + | <ref>http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/terp/lanost.html</ref> | ||
| + | Lanosterol and Cycloartenol Biosynthesis | ||
| + | INternational Union of Biochemistry and Molecular Biology 2000. | ||
| + | |||
{{Steroids}} | {{Steroids}} | ||
[[Category:Life sciences]] | [[Category:Life sciences]] | ||
| − | {{credit|Steroid|120869621|Steroid_hormone|122162270}} | + | {{credit|Steroid|120869621|Steroid_hormone|122162270|Anabolic_steroid|122867717}} |
Revision as of 00:29, 15 April 2007
A steroid is any of a group of naturally or synthetic, fat-soluble, organic compounds belonging to the class of lipids and characterized by a molecular core of four fused rings totaling 17 carbon atoms: three fused six-carbon rings and one five-carbon ring. The type of steroid is determined by the three-dimensional configuration and the type of additional side chains and rings.
Steroids are ubiquitous in nature, being found in animals, plants, fungi, and protozoa, and they perform many biological functions, including involvement in cell membranes and serving as hormones. Steorids include the sterols, bile acids, adrenal and sex hormones. Cholesterol is the most abundant steorid in mammalian cells.
Chemistry
Steroids belong to the class of biochemicals called lipids. Along with proteins, nucleic acids, and carbohydrates, lipids are one of the major classes of biologically important molecules (or biomolecules). They are water-insoluble, organic compounds that are highly soluble in nonpolar organic solvents. In addition to steroids, other lipids include fatty acids, glycerides (such as triglycerides or fat), phospholipids, and more complex lipid derivatives such as glycolipids (sugar-linked lipids).
A steroid is a lipid characterized by a carbon skeleton with four fused rings known as a cyclopentanoperhydrophenanthrene ring system. There are three fused six-carbon rings (cyclohexane) and a five-carbon ring (cyclopentane), fused such that there are a total of 17 carbon attoms in the molecular nucleus. Different steroids vary in the functional groups attached to these rings. All steroids are derived either from the sterol lanosterol (animals and fungi) or the sterol cycloartenol (plants). Both sterols are derived from the cyclization of the triterpene squalene (IUBMB 2000).
Classification
Some of the common categories of steroids:
- Animal steroids
- Insect steroids
- Ecdysteroids such as ecdysterone
- Vertebrate steroids
- Sex steroids are a subset of sex hormones that produce sex differences or support reproduction. They include androgens, estrogens, and progestagens.
- Corticosteroids include glucocorticoids and mineralocorticoids. Glucocorticoids regulate many aspects of metabolism and immune function, whereas mineralocorticoids help maintain blood volume and control renal excretion of electrolytes.
- Anabolic steroids are a class of steroids that interact with androgen receptors to increase muscle and bone synthesis. There are natural and synthetic anabolic steroids. These are the steroids used by athletes to increase performance.
- Cholesterol which modulates the fluidity of cell membranes and is the principle constituent of the plaques implicated in atherosclerosis.
- Insect steroids
- Plant steroids
- Phytosterols
- Brassinosteroids
- Fungus steroids
- Ergosterols
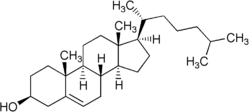
Cholesterol
Cholesterol is a sterol lipid (a combination steroid and alcohol) with the chemical formula C27H45OH. It is found in the cell membranes of all human body tissues, and transported in the blood plasma of all animals. Lesser amounts of cholesterol are also found in plant membranes.
Cholesterol is an important component of cell membranes, which enhances their fluidity. Cholesterol also aids in the manufacture of bile (which helps digest fats), and is also important for the metabolism of fat-soluble vitamins.
Steroid hormones
Cholesterol is an important precursor of the steroid hormones. Steroid hormones are steroids which act as hormones. Mammalian steroid hormones can be grouped into five groups by the receptors to which they bind: glucocorticoids, mineralocorticoids, androgens, estrogens, and progestagens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors, though technically sterols rather than steroids. Steroid hormones produce their physiological effects by binding to steroid hormone receptor proteins, which causes changes in gene transcription and cell function. These hormones include:
- Androgens (such as testosterone) are responsible for the development of male secondary sex characteristics.
- Glucocorticoids enable animals to respond to stress. They regulate many aspects of metabolism and immune function, and are often prescribed by doctors to reduce inflammatory conditions like asthma and arthritis.
- Mineralocorticoids help maintain blood volume and control renal excretion of electrolytes.
- Estrogens and progestagens are two classes of sex steroids, a subset of the hormones that produce sex differences or support reproduction.
The natural steroid hormones are generally synthesized from cholesterol in the gonads and adrenal glands. These forms of hormones are lipids. They can enter the cell membrane quite easily and enter right into the nuclei. Steroid hormones are generally carried in the blood bound to specific carrier proteins such as sex hormone binding globulin or corticosteroid binding globulin. Further conversions and catabolism occurs in the liver, other "peripheral" tissues, and in the target tissues.
Because steroids and sterols are lipid soluble, they can diffuse fairly freely from the blood through the cell membrane and into the cytoplasm of target cells. In the cytoplasm the steroid may or may not undergo an enzyme-mediated alteration such as reduction, hydroxylation, or aromatization. In the cytoplasm, the steroid binds to the specific receptor, a large metalloprotein. Upon steroid binding, many kinds of steroid receptor dimerize: two receptor subunits join together to form one functional DNA-binding unit that can enter the cell nucleus. In some of the hormone systems known, the receptor is associated with a heat shock protein which is released on the binding of the ligand, the hormone. Once in the nucleus, the steroid-receptor ligand complex binds to specific DNA sequences and induces transcription of its target genes.
Origin
Steroids include estrogen (U.S spelling) or oestrogen (UK spelling), progesterone and androgen. Oestrogen and progesterone are made primarily in the ovary and in the placenta during pregnancy and testosterone in the testes. Certain neurons and glia in the central nervous system (CNS) express the enzymes that are required for the local synthesis of pregnane neurosteroids, either de novo or from peripherally derived sources.
Anabolic steroids
Anabolic androgenic steroids or AAS are a class of natural and synthetic steroid hormones that promote cell growth and division, resulting in growth of several types of tissues, especially muscle and bone. Different anabolic androgenic steroids have varying combinations of androgenic and anabolic properties, and are often referred to in medical texts as AAS (anabolic/androgenic steroids). Anabolism is the metabolic process that builds larger molecules from smaller ones.
Anabolic steroids were first discovered in the early 1930s and have since been used for numerous medical purposes including stimulation of bone growth, appetite, puberty, and muscle growth. The most widespread use of anabolic steroids is their use for chronic wasting conditions, such as cancer and AIDS. Anabolic steroids can produce numerous physiological effects including increases in protein synthesis, muscle mass, strength, appetite and bone growth. Anabolic steroids have also been associated with numerous side effects when administered in excessive doses and these include elevated cholesterol (increase in LDL, decreased HDL levels), acne, elevated blood pressure, hepatotoxicity, and alterations in left ventricle morphology.
Today anabolic steroids are controversial because of their widespread use in competitive sports and their associated side effects. While there are numerous health issues associated with excessive anabolic steroid use, public understanding of the true risks remains limited. Anabolic steroids are controlled in a few countries including the United States, where they are listed as Schedule III in the Controlled Substances Act, as well as Canada and Britain who also have laws controlling their use and distribution.
External links
- Michael W. King's Medical Biochemistry. Steroids and retinoids are both terpenes which are hydrophobic, pass through cell membranes and bind to intracellular receptors. However, retinoic acid is not a steroid because is does not have the defining ring structure. See: Steroids and Related Hydrophobic Molecules.
- "Biochemistry" by Jeremy M. Berg, John L. Tymoczko and Lubert Stryer (2002) W. H. Freeman and Co. steroid topics in this
ReferencesISBN links support NWE through referral fees
- Agis-Balboa RC. et al. (2006). "Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis". Proc Natl Acad Sci U S A 103, 14602-14607. PMID 16984997
- Belelli D. and Lambert JJ. (2005). "Neurosteroids: endogenous regulators of the GABAA receptor". Nature Reviews Neuroscience 6, 565-575. PMID 15959466. Review
- Pinna G. et al. (2005). "Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake". Psychopharmacology (Berl) 186, 362-372. PMID 16432684. Review
- Dubrovsky BO. (2005). "Steroids, neuroactive steroids and neurosteroids in psychopathology". Prog Neuropsychopharmacol Biol Psychiatry 29, 169-192, PMID 15694225. Review
- Mellon SH. and Griffin LD. (2002) "Neurosteroids: biochemistry and clinical significance". Trends Endocrinol Metab 13, 35-43. PMID 11750861. Review
[1] Lanosterol and Cycloartenol Biosynthesis INternational Union of Biochemistry and Molecular Biology 2000.
| |||||||||||||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.