Difference between revisions of "Silicone" - New World Encyclopedia
| Line 6: | Line 6: | ||
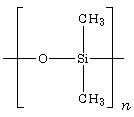
'''Silicones''' (more accurately called [[polymer]]ized [[siloxane]]s or '''polysiloxanes''') are mixed [[Inorganic compound|inorganic]]-[[Organic compound|organic]] polymers with the chemical formula [R<sub>2</sub>SiO]<sub>n</sub>, where [[functional group|R]] = organic groups such as [[methyl]], [[ethyl]], and [[phenyl]]. These materials consist of an inorganic [[silicon]]-[[oxygen]] backbone (...-Si-O-Si-O-Si-O-...) with organic side groups attached to the silicon atoms, which are four-coordinate. In some cases organic side groups can be used to link two or more of these -Si-O- backbones together. By varying the -Si-O- chain lengths, side groups, and [[Cross-link|crosslinking]], silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxane is linear [[polydimethylsiloxane]] (PDMS), a [[silicone oil]]. The second largest group of silicone materials is based on [[silicone resin]]s, which are formed by branched and cage-like oligosiloxanes. | '''Silicones''' (more accurately called [[polymer]]ized [[siloxane]]s or '''polysiloxanes''') are mixed [[Inorganic compound|inorganic]]-[[Organic compound|organic]] polymers with the chemical formula [R<sub>2</sub>SiO]<sub>n</sub>, where [[functional group|R]] = organic groups such as [[methyl]], [[ethyl]], and [[phenyl]]. These materials consist of an inorganic [[silicon]]-[[oxygen]] backbone (...-Si-O-Si-O-Si-O-...) with organic side groups attached to the silicon atoms, which are four-coordinate. In some cases organic side groups can be used to link two or more of these -Si-O- backbones together. By varying the -Si-O- chain lengths, side groups, and [[Cross-link|crosslinking]], silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxane is linear [[polydimethylsiloxane]] (PDMS), a [[silicone oil]]. The second largest group of silicone materials is based on [[silicone resin]]s, which are formed by branched and cage-like oligosiloxanes. | ||
| + | |||
| + | ==Chemical terminology== | ||
| + | Silicone is often mistakenly referred to as "silicon." Although silicones contain [[silicon]] atoms, they are not made up exclusively of silicon, and have completely different physical characteristics from elemental silicon. | ||
| + | |||
| + | The word "silicone" is derived from ''[[ketone]]''. Dimethylsilicone and dimethyl ketone (a.k.a. [[acetone]]) have analogous formulas, thus it was surmised (incorrectly) that they have analogous structures. The same terminology is used for compounds such as [[silane]] (an analogue of [[methane]]). A true ''silicone group'' with a double bond between oxygen and silicon (see figure) does not exist in nature; chemists find that the silicon atom forms a single bond with each of two oxygen atoms, rather than a double bond to a single atom. Polysiloxanes are called "silicone" due to early mistaken assumptions about their structure. | ||
| + | |||
| + | ==Synthesis== | ||
| + | Silicones are synthesized from [[chlorosilane]]s, [[tetraethoxysilane]], and related compounds. In the case of PDMS, the starting material is dimethylchlorosilane, which reacts with [[water]] as follows: | ||
| + | |||
| + | :n [Si(CH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>] + n [H<sub>2</sub>O] → [Si(CH<sub>3</sub>)<sub>2</sub>O]<sub>n</sub> + 2n HCl | ||
| + | |||
| + | During polymerization, this reaction evolves potentially hazardous [[hydrogen chloride]] gas. For medical uses, a process was developed where the chlorine atoms in the silane precursor were replaced with acetate groups, so that the reaction product of the final curing process is nontoxic [[acetic acid]] (vinegar). As a side effect, the curing process is also much slower in this case. This is the chemistry used in many consumer applications, such as silicone [[caulk]] and [[adhesive]]s. | ||
| + | |||
| + | Silane precursors with more acid-forming groups and fewer methyl groups, such as methyltrichlorosilane, can be used to introduce [[Branching (chemistry)|branches]] or [[cross-link]]s in the polymer chain. Ideally, each molecule of such a compound becomes a branch point. This can be used to produce hard [[silicone resin]]s. Similarly, precursors with three methyl groups can be used to limit molecular weight, since each such molecule has only one reactive site and so forms the end of a siloxane chain. | ||
| + | |||
| + | Modern silicone resins are made with [[tetraethoxysilane]], which reacts in a more mild and controllable manner than chlorosilanes. | ||
==Properties== | ==Properties== | ||
| + | |||
Some of the most useful properties of silicone include: | Some of the most useful properties of silicone include: | ||
# Thermal stability (Constancy of properties over a wide operating range of –100 to 250°C) | # Thermal stability (Constancy of properties over a wide operating range of –100 to 250°C) | ||
| Line 18: | Line 35: | ||
# Low toxicity | # Low toxicity | ||
# High gas permeability: at room temperature (25°C) the permeability of silicone rubber for gases like oxygen is approximately 400 times that of butyl rubber, making silicone useful for medical applications (though precluding it from applications where gas-tight seals are necessary). | # High gas permeability: at room temperature (25°C) the permeability of silicone rubber for gases like oxygen is approximately 400 times that of butyl rubber, making silicone useful for medical applications (though precluding it from applications where gas-tight seals are necessary). | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Versatile Applications== | ==Versatile Applications== | ||
| Line 38: | Line 44: | ||
Image:Kieselsaeure380m2prog.jpg|[[Silicon dioxide]] (silica) used in the manufacture of all silicones. This is what remains when one burns silicone; burning silicone caulking or foam produces silica (as well as char) as a white powder–silica fume. | Image:Kieselsaeure380m2prog.jpg|[[Silicon dioxide]] (silica) used in the manufacture of all silicones. This is what remains when one burns silicone; burning silicone caulking or foam produces silica (as well as char) as a white powder–silica fume. | ||
</gallery> | </gallery> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Uses of Silicone== | == Uses of Silicone== | ||
===Moldmaking Material=== | ===Moldmaking Material=== | ||
Two-Part silicone systems are also used to create rubber molds which can be used for production casting of resins, foams, rubber and low-temp alloys. A mold made of silicone generally requires little or no mold release or surface preparation as most materials do not adhere to moldmaking silicone. | Two-Part silicone systems are also used to create rubber molds which can be used for production casting of resins, foams, rubber and low-temp alloys. A mold made of silicone generally requires little or no mold release or surface preparation as most materials do not adhere to moldmaking silicone. | ||
| + | |||
===[[Sealant]]s in building construction and maintenance=== | ===[[Sealant]]s in building construction and maintenance=== | ||
One-part silicone sealants are in common use to seal gaps, joints and crevices in buildings. Silicones are abundantly available for this purpose, in professional, as well as retail grades. One-part silicones cure by absorbing atmospheric moisture, which helps in the professional installation. To make a very smooth silicone seal, apart from masking the edges with tape, if practical, professional caulkers often wet wooden spoons and assorted, hand-crafted wooden tools, which they soak in water with diluted dishsoap. The silicone will not stick to wet, soapy wood, which makes this ideal for creating a perfectly smooth surface. Do-it-yourselfers typically use a moistened finger to trace neat beads into silicone caulking. | One-part silicone sealants are in common use to seal gaps, joints and crevices in buildings. Silicones are abundantly available for this purpose, in professional, as well as retail grades. One-part silicones cure by absorbing atmospheric moisture, which helps in the professional installation. To make a very smooth silicone seal, apart from masking the edges with tape, if practical, professional caulkers often wet wooden spoons and assorted, hand-crafted wooden tools, which they soak in water with diluted dishsoap. The silicone will not stick to wet, soapy wood, which makes this ideal for creating a perfectly smooth surface. Do-it-yourselfers typically use a moistened finger to trace neat beads into silicone caulking. | ||
| Line 63: | Line 65: | ||
Silicone rubber is also used to make utensils (notably [[spatula]]s) and bakeware. | Silicone rubber is also used to make utensils (notably [[spatula]]s) and bakeware. | ||
| − | [[Silicone resin]]s are used in heat-resistant dishware. | + | [[Silicone resin]]s are used in heat-resistant dishware. These often resemble [[ceramic]] items, but are much less brittle, making them popular for use with babies. |
===Electronic components=== | ===Electronic components=== | ||
| − | Electronic components are sometimes protected from environmental | + | |
| + | Electronic components are sometimes protected from environmental influences by [[resin casting|enclosing]] them in silicone. This increases their stability against mechanical shock, radiation, and vibration. Silicones are selected over [[polyurethane]] or [[epoxy]] encapsulation when a wide operating temperature range is required (−150 to 600°F). Silicones also have the advantage of little exothermic heat rise during cure, low toxicity, good electrical properties and high purity. Therefore they are used when durability and high performance are demanded of components under demanding conditions, as for satellites in space. Silicones, however, are relatively expensive and can be attacked by solvents. | ||
===Hearing aids=== | ===Hearing aids=== | ||
| − | Silicone is a common material | + | |
| + | Silicone is a common material used in molds for behind-the-ear style [[hearing aids]]. It has excellent sealing properties, making it an ideal choice for patients with profound hearing losses needing high-powered hearing aids. | ||
===Menstrual cups=== | ===Menstrual cups=== | ||
| − | A [[menstrual cup]] is a type of cup or barrier worn inside the vagina during menstruation to collect menstrual fluid. Menstrual cups are often made of silicone for | + | |
| + | A [[menstrual cup]] is a type of cup or barrier worn inside the vagina during menstruation to collect menstrual fluid. Menstrual cups are often made of silicone for durability and reusability. | ||
===Silicone breast implants=== | ===Silicone breast implants=== | ||
| − | + | ||
Controversy developed in the 1980s and 1990s around claims that the silicone gel in [[Breast implant#Silicone gel implants|breast implants]] was responsible for a number of systemic health problems, including [[autoimmune diseases]] and cancer. Multiple lawsuits claiming injury from implants resulted in the 1998 [[bankruptcy]] of [[Dow Corning]] and a moratorium on the use of silicone implants for breast augmentation in the US and Canada pending study. However, multiple studies and expert review panels performed worldwide since then have consistently concluded that women with silicone breast implants are no more likely to develop systemic illness than women without breast implants. In 2006 both [[Health Canada]] and the US [[Food and Drug Administration|FDA]] adopted positions similar to other countries in permitting the use of silicone implants for cosmetic breast augmentation in their respective countries. | Controversy developed in the 1980s and 1990s around claims that the silicone gel in [[Breast implant#Silicone gel implants|breast implants]] was responsible for a number of systemic health problems, including [[autoimmune diseases]] and cancer. Multiple lawsuits claiming injury from implants resulted in the 1998 [[bankruptcy]] of [[Dow Corning]] and a moratorium on the use of silicone implants for breast augmentation in the US and Canada pending study. However, multiple studies and expert review panels performed worldwide since then have consistently concluded that women with silicone breast implants are no more likely to develop systemic illness than women without breast implants. In 2006 both [[Health Canada]] and the US [[Food and Drug Administration|FDA]] adopted positions similar to other countries in permitting the use of silicone implants for cosmetic breast augmentation in their respective countries. | ||
===Firestops=== | ===Firestops=== | ||
| − | Silicone foams have been used in North American as well as the Israeli [[Dimona]] [[nuclear reactor]] buildings in an attempt to [[firestop]] openings within fire-resistance rated wall and floor assemblies to prevent the spread of flames and smoke from one room to another. (The Israelis wisely switched to the somewhat more expensive yet much safer "[[elastomer]]" version of this product, which avoids most safety concerns associated with the foamed version.) Silicone foam firestops have been the subject of serious controversy and press attention due to lack of proper [[bounding]] and smoke development due to the pyrolysis of combustible components within the foam, [[hydrogen]] gas escape, shrinkage and cracking. These problems | + | |
| + | Silicone foams have been used in North American as well as the Israeli [[Dimona]] [[nuclear reactor]] buildings in an attempt to [[firestop]] openings within fire-resistance rated wall and floor assemblies to prevent the spread of flames and smoke from one room to another. (The Israelis wisely switched to the somewhat more expensive yet much safer "[[elastomer]]" version of this product, which avoids most safety concerns associated with the foamed version.) | ||
| + | |||
| + | Silicone foam firestops have been the subject of serious controversy and press attention due to lack of proper [[bounding]] and smoke development due to the pyrolysis of combustible components within the foam, [[hydrogen]] gas escape, shrinkage and cracking. These problems were exposed by [[Gerald W. Brown]], leading to a large number of reportable events among licensees (operators of [[nuclear power plant]]s) of the [[Nuclear Regulatory Commission]] (NRC). | ||
| + | |||
| + | When properly installed, silicone foam firestops can be fabricated for building code compliance. Advantages include flexibility and high [[dielectric]] strength. Disadvantages include poor bounding, combustibility (hard to extinguish) and significant smoke development. | ||
===Personal care products=== | ===Personal care products=== | ||
| − | Silicones are used as ingredients in some leave-in [[hair conditioner]] products. | + | |
| + | Silicones are used as ingredients in some leave-in [[hair conditioner]] products. These formulations utilize silicone's water resistance to prevent humidity from entering a dry hair shaft and ruining the style. | ||
===Dry cleaning=== | ===Dry cleaning=== | ||
| − | Liquid silicone can be used as a [[dry cleaning]] [[solvent]]. | + | |
| + | Liquid silicone can be used as a [[dry cleaning]] [[solvent]]. Touted as an "environmentally friendly" alternative to the traditional [[perchloroethylene]] (or perc) solvent, the decamethylpentacyclosiloxane (D5) process has been patented by the company [[GreenEarth Cleaning]]. The solvent degrades into sand and trace amounts of water and CO2, and waste produced from the D5 drycleaning process is nontoxic and nonhazardous. This significantly reduces the environmental impact of a typically high-polluting industry. | ||
| + | |||
Additionally, liquid silicone is chemically inert, meaning it does not react with fabrics or dyes during the cleaning process. This reduces the amount of fading and shrinking that most dry-cleaned garments experience. | Additionally, liquid silicone is chemically inert, meaning it does not react with fabrics or dyes during the cleaning process. This reduces the amount of fading and shrinking that most dry-cleaned garments experience. | ||
Revision as of 18:06, 22 August 2007
- Not to be confused with the element silicon.
Silicones (more accurately called polymerized siloxanes or polysiloxanes) are mixed inorganic-organic polymers with the chemical formula [R2SiO]n, where R = organic groups such as methyl, ethyl, and phenyl. These materials consist of an inorganic silicon-oxygen backbone (...-Si-O-Si-O-Si-O-...) with organic side groups attached to the silicon atoms, which are four-coordinate. In some cases organic side groups can be used to link two or more of these -Si-O- backbones together. By varying the -Si-O- chain lengths, side groups, and crosslinking, silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxane is linear polydimethylsiloxane (PDMS), a silicone oil. The second largest group of silicone materials is based on silicone resins, which are formed by branched and cage-like oligosiloxanes.
Chemical terminology
Silicone is often mistakenly referred to as "silicon." Although silicones contain silicon atoms, they are not made up exclusively of silicon, and have completely different physical characteristics from elemental silicon.
The word "silicone" is derived from ketone. Dimethylsilicone and dimethyl ketone (a.k.a. acetone) have analogous formulas, thus it was surmised (incorrectly) that they have analogous structures. The same terminology is used for compounds such as silane (an analogue of methane). A true silicone group with a double bond between oxygen and silicon (see figure) does not exist in nature; chemists find that the silicon atom forms a single bond with each of two oxygen atoms, rather than a double bond to a single atom. Polysiloxanes are called "silicone" due to early mistaken assumptions about their structure.
Synthesis
Silicones are synthesized from chlorosilanes, tetraethoxysilane, and related compounds. In the case of PDMS, the starting material is dimethylchlorosilane, which reacts with water as follows:
- n [Si(CH3)2Cl2] + n [H2O] → [Si(CH3)2O]n + 2n HCl
During polymerization, this reaction evolves potentially hazardous hydrogen chloride gas. For medical uses, a process was developed where the chlorine atoms in the silane precursor were replaced with acetate groups, so that the reaction product of the final curing process is nontoxic acetic acid (vinegar). As a side effect, the curing process is also much slower in this case. This is the chemistry used in many consumer applications, such as silicone caulk and adhesives.
Silane precursors with more acid-forming groups and fewer methyl groups, such as methyltrichlorosilane, can be used to introduce branches or cross-links in the polymer chain. Ideally, each molecule of such a compound becomes a branch point. This can be used to produce hard silicone resins. Similarly, precursors with three methyl groups can be used to limit molecular weight, since each such molecule has only one reactive site and so forms the end of a siloxane chain.
Modern silicone resins are made with tetraethoxysilane, which reacts in a more mild and controllable manner than chlorosilanes.
Properties
Some of the most useful properties of silicone include:
- Thermal stability (Constancy of properties over a wide operating range of –100 to 250°C)
- The ability to repel water and form watertight seals
- Excellent resistance to oxygen, ozone, and sunlight
- Flexibility
- Good electrical insulation
- Anti-adhesive
- Low chemical reactivity
- Low toxicity
- High gas permeability: at room temperature (25°C) the permeability of silicone rubber for gases like oxygen is approximately 400 times that of butyl rubber, making silicone useful for medical applications (though precluding it from applications where gas-tight seals are necessary).
Versatile Applications
Self-levelling silicone firestop installation in mechanical service penetration in 2 hour rated concrete floor.
- Foamfixer.jpg
Silicone "foamfixer" pump used to apply silicone foam firestop materials.
- Kieselsaeure380m2prog.jpg
Silicon dioxide (silica) used in the manufacture of all silicones. This is what remains when one burns silicone; burning silicone caulking or foam produces silica (as well as char) as a white powder–silica fume.
Uses of Silicone
Moldmaking Material
Two-Part silicone systems are also used to create rubber molds which can be used for production casting of resins, foams, rubber and low-temp alloys. A mold made of silicone generally requires little or no mold release or surface preparation as most materials do not adhere to moldmaking silicone.
Sealants in building construction and maintenance
One-part silicone sealants are in common use to seal gaps, joints and crevices in buildings. Silicones are abundantly available for this purpose, in professional, as well as retail grades. One-part silicones cure by absorbing atmospheric moisture, which helps in the professional installation. To make a very smooth silicone seal, apart from masking the edges with tape, if practical, professional caulkers often wet wooden spoons and assorted, hand-crafted wooden tools, which they soak in water with diluted dishsoap. The silicone will not stick to wet, soapy wood, which makes this ideal for creating a perfectly smooth surface. Do-it-yourselfers typically use a moistened finger to trace neat beads into silicone caulking.
Similar methods work for urethane caulking, against which silicones compete quite heavily. White silicones frequently turn slightly yellow over time.
The strength and reliability of silicone rubber is widely acknowledged in the construction industry. Automotive body manufacturing plants and paint shops must avoid the presence of all silicones, as a mere hint of its presence in any form can cause severe failures in automotive paints. Vendors and contractors in such plants are often requested to verify in writing that they will not bring any silicones into the plant.
In the plumbing and automotive fields, silicone grease is often used as a lubricant. Automotive spark plug wires are often insulated by multiple layers of silicone. Such construction prevents spark from jumping to an adjacent wire, causing a misfire. It also minimizes RFI, which can interfere with an engine management computer. In plumbing, the grease is typically applied to O-rings in faucets and valves. In the automotive field, silicone grease is typically used as a lubricant for brake components since it is stable at high temperatures, is not water-soluble, and is far less likely than other lubricants to foul brake pads.
Cooking applications
Silicone is also impregnated into parchment paper and used as a non-stick material for applications such as baking and steaming. The silicone also makes the paper heat- and grease-resistant. This allows the paper to line cookie sheets and act as a replacement for greasing, thereby speeding mass production of baked goods. It is also commonly used in pouch cooking, where ingredients are sealed into a container made of parchment paper and allowed to steam.
Silicone rubber is also used to make utensils (notably spatulas) and bakeware.
Silicone resins are used in heat-resistant dishware. These often resemble ceramic items, but are much less brittle, making them popular for use with babies.
Electronic components
Electronic components are sometimes protected from environmental influences by enclosing them in silicone. This increases their stability against mechanical shock, radiation, and vibration. Silicones are selected over polyurethane or epoxy encapsulation when a wide operating temperature range is required (−150 to 600°F). Silicones also have the advantage of little exothermic heat rise during cure, low toxicity, good electrical properties and high purity. Therefore they are used when durability and high performance are demanded of components under demanding conditions, as for satellites in space. Silicones, however, are relatively expensive and can be attacked by solvents.
Hearing aids
Silicone is a common material used in molds for behind-the-ear style hearing aids. It has excellent sealing properties, making it an ideal choice for patients with profound hearing losses needing high-powered hearing aids.
Menstrual cups
A menstrual cup is a type of cup or barrier worn inside the vagina during menstruation to collect menstrual fluid. Menstrual cups are often made of silicone for durability and reusability.
Silicone breast implants
Controversy developed in the 1980s and 1990s around claims that the silicone gel in breast implants was responsible for a number of systemic health problems, including autoimmune diseases and cancer. Multiple lawsuits claiming injury from implants resulted in the 1998 bankruptcy of Dow Corning and a moratorium on the use of silicone implants for breast augmentation in the US and Canada pending study. However, multiple studies and expert review panels performed worldwide since then have consistently concluded that women with silicone breast implants are no more likely to develop systemic illness than women without breast implants. In 2006 both Health Canada and the US FDA adopted positions similar to other countries in permitting the use of silicone implants for cosmetic breast augmentation in their respective countries.
Firestops
Silicone foams have been used in North American as well as the Israeli Dimona nuclear reactor buildings in an attempt to firestop openings within fire-resistance rated wall and floor assemblies to prevent the spread of flames and smoke from one room to another. (The Israelis wisely switched to the somewhat more expensive yet much safer "elastomer" version of this product, which avoids most safety concerns associated with the foamed version.)
Silicone foam firestops have been the subject of serious controversy and press attention due to lack of proper bounding and smoke development due to the pyrolysis of combustible components within the foam, hydrogen gas escape, shrinkage and cracking. These problems were exposed by Gerald W. Brown, leading to a large number of reportable events among licensees (operators of nuclear power plants) of the Nuclear Regulatory Commission (NRC).
When properly installed, silicone foam firestops can be fabricated for building code compliance. Advantages include flexibility and high dielectric strength. Disadvantages include poor bounding, combustibility (hard to extinguish) and significant smoke development.
Personal care products
Silicones are used as ingredients in some leave-in hair conditioner products. These formulations utilize silicone's water resistance to prevent humidity from entering a dry hair shaft and ruining the style.
Dry cleaning
Liquid silicone can be used as a dry cleaning solvent. Touted as an "environmentally friendly" alternative to the traditional perchloroethylene (or perc) solvent, the decamethylpentacyclosiloxane (D5) process has been patented by the company GreenEarth Cleaning. The solvent degrades into sand and trace amounts of water and CO2, and waste produced from the D5 drycleaning process is nontoxic and nonhazardous. This significantly reduces the environmental impact of a typically high-polluting industry.
Additionally, liquid silicone is chemically inert, meaning it does not react with fabrics or dyes during the cleaning process. This reduces the amount of fading and shrinking that most dry-cleaned garments experience.
See also
- Breast implant
- Dental dam
- Dry cleaning
- Firestop
- Nuclear Reactor
- Parchment
- Sealant
- Silicon
External links
- Silicone Polymers (Virtual Chembook, Elmhurst College) Retrieved August 22, 2007.
- [http://www.ontla.on.ca/web/committee-proceedings/committee_transcripts_details.do?locale=en&Date=1997-10-27&ParlCommID=828&BillID=&Business=Hydro+Stakeholders
Committee Transcripts: Select Committee on Hydro Nuclear Affairs - October 27, 1997 - Hydro Stakeholders.] Legislative Assembly of Ontario. Retrieved August 22, 2007.
- NIRS Reactorwatch U.S. Nuclear Regulatory Commission. Retrieved August 22, 2007.
- Flammable 'Firestops' Used in CANDU Reactors. Press release of U.S. Representative Ed Markey's Statements. Retrieved August 22, 2007.
- Potential Problems with Silicone Foam Fire Barrier Penetration Seals. U.S. Nuclear Regulatory Commission. Retrieved August 22, 2007.
- Silicones Science Online. Centre Européen des Silicones (CES). Retrieved August 22, 2007.
- The Basics of Silicon Chemistry. Dow Corning. Retrieved August 22, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.