Difference between revisions of "Refrigeration" - New World Encyclopedia
| Line 9: | Line 9: | ||
The use of [[ice]] to preserve food by refrigeration goes back to prehistoric times.<ref name=Lawrence>{{PDF|[http://bama.ua.edu/~chem/seminars/student_seminars/spring03/papers-s03/lawrence-s03.pdf "Refrigeration fundamentals throughout history"]|72.9 [[Kibibyte|KiB]]<!-- application/pdf, 74724 bytes —>}}</ref><ref name=ASHRAE>{{PDF|[http://www.ashrae.org/content/ASHRAE/ASHRAE/ArticleAltFormat/200651081623_347.pdf "Air conditioning and refrigeration chronology"]|265 KiB<!-- application/pdf, 271833 bytes —>}}</ref> Through the ages, the seasonal harvesting of snow and ice was a regular practice of most of the ancient cultures: Chinese, Hebrews, Greeks, Romans, Persians. Ice and snow were stored in caves or dugouts lined with straw or other insulating materials. The Persians stored ice in pits called [[Yakhchal]]s. Rationing of the ice allowed the preservation of foods over the hot periods. This practice worked well down through the centuries, with [[Icehouse (building)|icehouse]]s remaining in use into the twentieth century. | The use of [[ice]] to preserve food by refrigeration goes back to prehistoric times.<ref name=Lawrence>{{PDF|[http://bama.ua.edu/~chem/seminars/student_seminars/spring03/papers-s03/lawrence-s03.pdf "Refrigeration fundamentals throughout history"]|72.9 [[Kibibyte|KiB]]<!-- application/pdf, 74724 bytes —>}}</ref><ref name=ASHRAE>{{PDF|[http://www.ashrae.org/content/ASHRAE/ASHRAE/ArticleAltFormat/200651081623_347.pdf "Air conditioning and refrigeration chronology"]|265 KiB<!-- application/pdf, 271833 bytes —>}}</ref> Through the ages, the seasonal harvesting of snow and ice was a regular practice of most of the ancient cultures: Chinese, Hebrews, Greeks, Romans, Persians. Ice and snow were stored in caves or dugouts lined with straw or other insulating materials. The Persians stored ice in pits called [[Yakhchal]]s. Rationing of the ice allowed the preservation of foods over the hot periods. This practice worked well down through the centuries, with [[Icehouse (building)|icehouse]]s remaining in use into the twentieth century. | ||
| − | In the 16th century, the discovery of chemical refrigeration was one of the first steps toward artificial means of refrigeration. [[Sodium nitrate]] or [[potassium nitrate]], when added to water, lowered the water temperature and created a sort of refrigeration bath for cooling substances. In France, cold drinks and liqueurs were produced by spinning long-necked bottles in water with dissolved saltpeter | + | In the 16th century, the discovery of chemical refrigeration was one of the first steps toward artificial means of refrigeration. [[Sodium nitrate]] or [[potassium nitrate]], when added to water, lowered the water temperature and created a sort of refrigeration bath for cooling substances. In France, cold drinks and liqueurs were produced by spinning long-necked bottles in water with dissolved saltpeter. |
During the first half of the 19th century, ice harvesting had become big business in America. New Englander Frederic Tudor (1783-1864), who became known as the "Ice King," worked on developing better [[Thermal insulation|insulation]] products for the long distance shipment of ice, especially to the tropics. | During the first half of the 19th century, ice harvesting had become big business in America. New Englander Frederic Tudor (1783-1864), who became known as the "Ice King," worked on developing better [[Thermal insulation|insulation]] products for the long distance shipment of ice, especially to the tropics. | ||
| Line 21: | Line 21: | ||
In 1820, the British scientist Michael Faraday (1791-1867) liquified [[ammonia]] and other gases by using high pressures and low temperatures. | In 1820, the British scientist Michael Faraday (1791-1867) liquified [[ammonia]] and other gases by using high pressures and low temperatures. | ||
| − | An American living in Great Britain, Jacob Perkins (1766-1849), obtained the first patent for a [[vapor-compression refrigeration]] system in 1834. Perkins built a prototype system and it actually worked, although it did not succeed commercially.<ref | + | An American living in Great Britain, Jacob Perkins (1766-1849), obtained the first patent for a [[vapor-compression refrigeration]] system in 1834. Perkins built a prototype system and it actually worked, although it did not succeed commercially.<ref>Burstall, Aubrey F. 1965. "A History of Mechanical Engineering". The MIT Press. ISBN 0-262-52001-X. |
| − | + | </ref> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | In 1842, an American physician, John Gorrie (1803-1855), designed the first system for refrigerating water to produce ice. He also conceived the idea of using his refrigeration system to cool the air for comfort in homes and hospitals (i.e., air-conditioning). His system compressed air, then partially cooled the hot compressed air with water before allowing it to expand while doing part of the work required to drive the air [[gas compressor|compressor]]. That [[isentropic]] expansion cooled the air to a temperature low enough to freeze water and produce ice, or to flow "through a pipe for effecting refrigeration otherwise" as stated in his patent granted by the [[U.S. Patent Office]] in 1851. | + | In 1842, an American physician, John Gorrie (1803-1855), designed the first system for refrigerating water to produce ice. He also conceived the idea of using his refrigeration system to cool the air for comfort in homes and hospitals (i.e., air-conditioning). His system compressed air, then partially cooled the hot compressed air with water before allowing it to expand while doing part of the work required to drive the air [[gas compressor|compressor]]. That [[isentropic]] expansion cooled the air to a temperature low enough to freeze water and produce ice, or to flow "through a pipe for effecting refrigeration otherwise" as stated in his patent granted by the [[U.S. Patent Office]] in 1851. Gorrie built a working prototype, but his system was a commercial failure. |
Alexander Twining began experimenting with vapor-compression refrigeration in 1848 and obtained patents in 1850 and 1853. He is credited with having initiated commercial refrigeration in the United States by 1856. | Alexander Twining began experimenting with vapor-compression refrigeration in 1848 and obtained patents in 1850 and 1853. He is credited with having initiated commercial refrigeration in the United States by 1856. | ||
| Line 40: | Line 34: | ||
Thaddeus Lowe (1832-1913), an American balloonist from the Civil War, had experimented over the years with the properties of gases. One of his mainstay enterprises was the high-volume production of [[hydrogen]] gas. He also held several patents on ice making machines. His "Compression Ice Machine" would revolutionize the cold storage industry. In 1869 he and other investors purchased an old steamship onto which they loaded one of Lowe’s refrigeration units and began shipping fresh fruit from New York to the Gulf Coast area, and fresh meat from Galveston, Texas back to New York. Because of Lowe’s lack of knowledge about shipping, the business was a costly failure, and it was difficult for the public to get used to the idea of being able to consume meat that had been so long out of the packing house. | Thaddeus Lowe (1832-1913), an American balloonist from the Civil War, had experimented over the years with the properties of gases. One of his mainstay enterprises was the high-volume production of [[hydrogen]] gas. He also held several patents on ice making machines. His "Compression Ice Machine" would revolutionize the cold storage industry. In 1869 he and other investors purchased an old steamship onto which they loaded one of Lowe’s refrigeration units and began shipping fresh fruit from New York to the Gulf Coast area, and fresh meat from Galveston, Texas back to New York. Because of Lowe’s lack of knowledge about shipping, the business was a costly failure, and it was difficult for the public to get used to the idea of being able to consume meat that had been so long out of the packing house. | ||
| − | Domestic refrigeration appeared about 1910. | + | Domestic refrigeration appeared about 1910. In 1923, Kelvinator made the first refrigerator with automatic temperature control<ref>[http://www.greatachievements.org/?id=3862Air Conditioning and Refrigeration History]</ref>. General Electric introduced some of the first hermetic units in 1928, called the Monitor Top<ref>[http://www.antiqueappliances.com/monitor_top_refrigerators.htm Monitor Top Refrigerators]</ref>. |
===Widespread commercial use=== | ===Widespread commercial use=== | ||
| Line 64: | Line 58: | ||
As of 1989, Freon was banned via the [[Montreal Protocol]] due to the negative effects it has on the [[ozone layer]]. The Montreal Protocol was ratified by most CFC producing and consuming nations in Montreal, Quebec, Canada in September 1987. | As of 1989, Freon was banned via the [[Montreal Protocol]] due to the negative effects it has on the [[ozone layer]]. The Montreal Protocol was ratified by most CFC producing and consuming nations in Montreal, Quebec, Canada in September 1987. | ||
| − | The tenets of the Montreal Protocol were put into effect in the [[United States]] via the [[Clean Air Act (1990)|Clean Air Act]] legislation in August 1988. The Clean Air Act was further amended in 1990. This was a direct result of a scientific report released in June 1974 by Rowland-Molina<ref>''Stratospheric sink for chlorofluoromethanes: | + | The tenets of the Montreal Protocol were put into effect in the [[United States]] via the [[Clean Air Act (1990)|Clean Air Act]] legislation in August 1988. The Clean Air Act was further amended in 1990. This was a direct result of a scientific report released in June 1974 by Rowland-Molina<ref>Molina, Mario J. & Rowland, F. S. 1974. ''Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone''. Department of Chemistry, [[University of California, Irvine]]. Nature 249, pp. 810-12.</ref>, detailing how [[chlorine]] in CFC and [[HCFC]] refrigerants adversely affected the ozone layer. This report prompted the [[FDA]] and [[EPA]] to ban CFCs as a propellant in 1978 (50% of CFC use at that time was for aerosol can propellant). |
*In January 1992, the EPA required that refrigerant be recovered from all automotive air conditioning systems during system service. | *In January 1992, the EPA required that refrigerant be recovered from all automotive air conditioning systems during system service. | ||
| Line 106: | Line 100: | ||
A ''refrigeration cycle'' describes the changes that take place in the refrigerant as it alternately absorbs and rejects heat as it circulates through a [[refrigerator]]. It is also applied to HVACR work, when describing the "process" of refrigerant flow through an HVACR unit, whether it is a packaged or split system. | A ''refrigeration cycle'' describes the changes that take place in the refrigerant as it alternately absorbs and rejects heat as it circulates through a [[refrigerator]]. It is also applied to HVACR work, when describing the "process" of refrigerant flow through an HVACR unit, whether it is a packaged or split system. | ||
| − | Heat naturally flows from hot to cold. [[mechanical work|Work]] is applied to cool a living space or storage volume by pumping heat from a lower temperature heat source into a higher temperature heat sink. [[Thermal insulation|Insulation]] is used to reduce the work and energy required to achieve and maintain a lower temperature in the cooled space. The operating principle of the refrigeration cycle was described mathematically by | + | Heat naturally flows from hot to cold. [[mechanical work|Work]] is applied to cool a living space or storage volume by pumping heat from a lower temperature heat source into a higher temperature heat sink. [[Thermal insulation|Insulation]] is used to reduce the work and energy required to achieve and maintain a lower temperature in the cooled space. The operating principle of the refrigeration cycle was described mathematically by Nicolas Léonard Sadi Carnot (1796-1832) in 1824 as a [[Carnot heat engine|heat engine]]. |
The most common types of refrigeration systems use the reverse-Rankine [[vapor-compression refrigeration]] cycle although [[absorption heat pump]]s are used in a minority of applications. | The most common types of refrigeration systems use the reverse-Rankine [[vapor-compression refrigeration]] cycle although [[absorption heat pump]]s are used in a minority of applications. | ||
| Line 126: | Line 120: | ||
The above discussion is based on the ideal vapour-compression refrigeration cycle, and does not take into account real-world effects like frictional pressure drop in the system, slight [[thermodynamic reversibility|thermodynamic irreversibility]] during the compression of the refrigerant vapour, or [[ideal gas|non-ideal gas]] behavior (if any). | The above discussion is based on the ideal vapour-compression refrigeration cycle, and does not take into account real-world effects like frictional pressure drop in the system, slight [[thermodynamic reversibility|thermodynamic irreversibility]] during the compression of the refrigerant vapour, or [[ideal gas|non-ideal gas]] behavior (if any). | ||
| − | More information about the design and performance of vapour-compression refrigeration systems is available in the classic "[[Perry's Chemical Engineers' Handbook]]".<ref> | + | More information about the design and performance of vapour-compression refrigeration systems is available in the classic "[[Perry's Chemical Engineers' Handbook]]".<ref>Perry, R.H. and Green, D.W. 1984. ''Perry's Chemical Engineers' Handbook''. McGraw Hill, Inc. ISBN 0070494797.</ref> |
==== Vapour absorption cycle ==== | ==== Vapour absorption cycle ==== | ||
| Line 190: | Line 184: | ||
* Stoecker, Wilbert F. and Jones, Jerold W. 1983. ''Refrigeration and Air Conditioning''. McGraw Hill Higher Education. ISBN 0070665915. | * Stoecker, Wilbert F. and Jones, Jerold W. 1983. ''Refrigeration and Air Conditioning''. McGraw Hill Higher Education. ISBN 0070665915. | ||
* Althouse, Andrew D., Carl H. Turnquist, and Alfred F. Bracciano. 2003. ''Modern Refrigeration and Air Conditioning''. Goodheart-Wilcox Publisher; 18 edition. ISBN 1590702808. | * Althouse, Andrew D., Carl H. Turnquist, and Alfred F. Bracciano. 2003. ''Modern Refrigeration and Air Conditioning''. Goodheart-Wilcox Publisher; 18 edition. ISBN 1590702808. | ||
| + | *Brain, Marshall. 2006. [http://home.howstuffworks.com/refrigerator4.htm "How Refrigeration Work"]. HowStuffWorks.com. Retrieved September 3, 2007. | ||
==External links== | ==External links== | ||
| − | |||
| − | |||
| − | |||
* [http://www.ashrae.org/ American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE)] | * [http://www.ashrae.org/ American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE)] | ||
* [http://www.ior.org.uk/ British Institute of Refrigeration] | * [http://www.ior.org.uk/ British Institute of Refrigeration] | ||
Revision as of 17:30, 3 September 2007
Refrigeration is the process of removing heat from an enclosed space, or from a substance, and discharging it elsewhere for the primary purpose of lowering the temperature of the enclosed space or substance and then maintaining that lower temperature. To satisfy the Second Law of Thermodynamics, mechanical work must be performed to accomplish this.
Historical applications
Ice harvesting
The use of ice to preserve food by refrigeration goes back to prehistoric times.[1][2] Through the ages, the seasonal harvesting of snow and ice was a regular practice of most of the ancient cultures: Chinese, Hebrews, Greeks, Romans, Persians. Ice and snow were stored in caves or dugouts lined with straw or other insulating materials. The Persians stored ice in pits called Yakhchals. Rationing of the ice allowed the preservation of foods over the hot periods. This practice worked well down through the centuries, with icehouses remaining in use into the twentieth century.
In the 16th century, the discovery of chemical refrigeration was one of the first steps toward artificial means of refrigeration. Sodium nitrate or potassium nitrate, when added to water, lowered the water temperature and created a sort of refrigeration bath for cooling substances. In France, cold drinks and liqueurs were produced by spinning long-necked bottles in water with dissolved saltpeter.
During the first half of the 19th century, ice harvesting had become big business in America. New Englander Frederic Tudor (1783-1864), who became known as the "Ice King," worked on developing better insulation products for the long distance shipment of ice, especially to the tropics.
First refrigeration systems
The first known method of artificial refrigeration was demonstrated by William Cullen (1710-1790) at the University of Glasgow in Scotland in 1748. Cullen used a pump to create a partial vacuum over a container of ethyl ether, which then boiled , absorbing heat from the surrounding air. The experiment even created a small amount of ice, but had no practical application at that time.
In 1805, American inventor Oliver Evans (1755-1819) designed but never built a refrigeration system based on the Vapor-compression refrigeration cycle rather than chemical solutions or volatile liquids such as ethyl ether.
In 1820, the British scientist Michael Faraday (1791-1867) liquified ammonia and other gases by using high pressures and low temperatures.
An American living in Great Britain, Jacob Perkins (1766-1849), obtained the first patent for a vapor-compression refrigeration system in 1834. Perkins built a prototype system and it actually worked, although it did not succeed commercially.[3]
In 1842, an American physician, John Gorrie (1803-1855), designed the first system for refrigerating water to produce ice. He also conceived the idea of using his refrigeration system to cool the air for comfort in homes and hospitals (i.e., air-conditioning). His system compressed air, then partially cooled the hot compressed air with water before allowing it to expand while doing part of the work required to drive the air compressor. That isentropic expansion cooled the air to a temperature low enough to freeze water and produce ice, or to flow "through a pipe for effecting refrigeration otherwise" as stated in his patent granted by the U.S. Patent Office in 1851. Gorrie built a working prototype, but his system was a commercial failure.
Alexander Twining began experimenting with vapor-compression refrigeration in 1848 and obtained patents in 1850 and 1853. He is credited with having initiated commercial refrigeration in the United States by 1856.
Soon after that, James Harrison(1816-1893), born in Scotland and subsequently emigrated to Australia, introduced commercial vapor-compression refrigeration to breweries and meat packing houses. By 1861, a dozen of his systems were in operation.
The first gas absorption refrigeration system using gaseous ammonia dissolved in water (referred to as "aqua ammonia") was developed by Ferdinand Carré of France in 1859 and patented in 1860. Due to the toxicity of ammonia, such systems were not developed for use in homes, but were used to manufacture ice for sale. The consumer public at that time still used the ice box with ice brought in from commercial suppliers, many of whom were still harvesting ice and storing it in an icehouse.
Thaddeus Lowe (1832-1913), an American balloonist from the Civil War, had experimented over the years with the properties of gases. One of his mainstay enterprises was the high-volume production of hydrogen gas. He also held several patents on ice making machines. His "Compression Ice Machine" would revolutionize the cold storage industry. In 1869 he and other investors purchased an old steamship onto which they loaded one of Lowe’s refrigeration units and began shipping fresh fruit from New York to the Gulf Coast area, and fresh meat from Galveston, Texas back to New York. Because of Lowe’s lack of knowledge about shipping, the business was a costly failure, and it was difficult for the public to get used to the idea of being able to consume meat that had been so long out of the packing house.
Domestic refrigeration appeared about 1910. In 1923, Kelvinator made the first refrigerator with automatic temperature control[4]. General Electric introduced some of the first hermetic units in 1928, called the Monitor Top[5].
Widespread commercial use
By the 1870s breweries had become the largest users of commercial refrigeration units, though some still relied on harvested ice. Though the ice-harvesting industry had grown immensely by the turn of the 20th century, pollution and sewage had begun to creep into natural ice making it a problem in the metropolitan suburbs. Eventually breweries began to complain of tainted ice. This raised demand for more modern and consumer-ready refrigeration and ice-making machines. In 1895 German engineer Carl von Linde (1842-1934) set up a large-scale process for the production of liquid air and eventually liquid oxygen for use in safe household refrigerators.
Refrigerated railroad cars were introduced in the 1840s for the short-run transportation of dairy products. In 1867 J.B. Sutherland of Detroit, Michigan patented the refrigerator car designed with ice tanks at either end of the car and ventilator flaps near the floor which would create a gravity draft of cold air through the car.
By 1900 the meat packing houses of Chicago had adopted ammonia-cycle commercial refrigeration. By 1914 almost every location used artificial refrigeration. The big meat packers, Armour, Swift, and Wilson, had purchased the most expensive units which they installed on train cars and in branch houses and storage facilities in the more remote distribution areas.
It was not until the middle of the 20th century that refrigeration units were designed for installation on tractor-trailer rigs (trucks). Refrigerated trucks are used to transport perishable goods, such as frozen foods, fruit and vegetables, and temperature-sensitive chemicals. Most modern refrigerators keep the temperature between -40 and +20 °C and have a maximum payload of around 24 000 kg. gross weight (in Europe)
Home and consumer use
With the invention of synthetic refrigerants like Freon, safer refrigerators were possible for home and consumer use. Freon is referred to as a CFC (Chlorofluorocarbon), halocarbon, or haloalkane.
Developed in the late 1920s, Freon is much less toxic than some of the refrigerants used earlier (i.e., methyl formate, ammonia, methyl chloride and sulfur dioxide) and Freon-12 has a boiling point of -22 °F (-30 °C). The intent was to provide refrigeration units for home use without the use of toxic refrigerants. At the same time the units needed to be made smaller which meant using refrigerants that could do more work with fewer parts in less space. A refrigerant like Freon answered that need.
The Freon patents were initially held by the automotive industry who used it for auto air-conditioning, but the product was far too useful to limit to automotive use. By 1930 Freon was available on the open market.
The Montreal Protocol
As of 1989, Freon was banned via the Montreal Protocol due to the negative effects it has on the ozone layer. The Montreal Protocol was ratified by most CFC producing and consuming nations in Montreal, Quebec, Canada in September 1987.
The tenets of the Montreal Protocol were put into effect in the United States via the Clean Air Act legislation in August 1988. The Clean Air Act was further amended in 1990. This was a direct result of a scientific report released in June 1974 by Rowland-Molina[6], detailing how chlorine in CFC and HCFC refrigerants adversely affected the ozone layer. This report prompted the FDA and EPA to ban CFCs as a propellant in 1978 (50% of CFC use at that time was for aerosol can propellant).
- In January 1992, the EPA required that refrigerant be recovered from all automotive air conditioning systems during system service.
- In July 1992, the EPA made illegal the venting of CFC and HCFC refrigerants.
- In June 1993, the EPA required that major leaks in refrigeration systems be fixed within 30 days. A major leak was defined as a leak rate that would equal 35% of the total refrigerant charge of the system (for industrial and commercial refrigerant systems), or 15% of the total refrigerant charge of the system (for all other large refrigerant systems), if that leak were to proceed for an entire year.
- In July 1993, the EPA instituted the Safe Disposal Requirements, requiring that all refrigerant systems be evacuated prior to retirement or disposal (no matter the size of the system), and putting the onus on the last person in the disposal chain to ensure that the refrigerant was properly captured.
- In August 1993, the EPA implemented reclamation requirements for refrigerant. If a refrigerant is to change ownership, it must be processed and tested to comply with the American Refrigeration Institute (ARI) standard 700-1993 (now ARI standard 700-1995) requirements for refrigerant purity.
- In November 1993, the EPA required that all refrigerant recovery equipment meet the standards of ARI 740-1993.
- In November 1995, the EPA also restricted the venting of HFC refrigerants. These contain no chlorine that can damage the ozone layer (and thus have an ODP (Ozone Depletion Potential) of zero), but still have a high global warming potential.
- In December 1995, CFC refrigerant importation and production in the US was banned.
It is currently planned to ban all HCFC refrigerant importation and production in the year 2030, although that will likely be accelerated.
Current applications of refrigeration
Probably the most widely-used current applications of refrigeration are for the air-conditioning of private homes and public buildings, and the refrigeration of foodstuffs in homes, restaurants and large storage warehouses. The use of refrigerators in our kitchens for the storage of fruits and vegetables has allowed us to add fresh salads to our diets year round, and to store fish and meats safely for long periods.
In commerce and manufacturing, there are many uses for refrigeration. Refrigeration is used to liquify gases like oxygen, nitrogen, propane and methane for example. In compressed air purification, it is used to condense water vapour from compressed air to reduce its moisture content. In oil refineries, chemical plants, and petrochemical plants, refrigeration is used to maintain certain processes at their required low temperatures (for example, in the alkylation of butenes and butane to produce a high octane gasoline component). Metal workers use refrigeration to temper steel and cutlery. In transporting temperature-sensitive foodstuffs and other materials by trucks, trains, airplanes and sea-going vessels, refrigeration is a necessity.
Dairy products are constantly in need of refrigeration, and it was only discovered in the past few decades that eggs needed to be refrigerated during shipment rather than waiting to be refrigerated after arrival at the grocery store. Meats, poultry and fish all must be kept in climate-controlled environments before being sold. Refrigeration also helps keep fruits and vegetables edible longer.
Methods of refrigeration
Methods of refrigeration can be classified as non-cyclic, cyclic and thermoelectric.
Non-cyclic refrigeration
In these methods, refrigeration can be accomplished by melting ice or by subliming dry ice. These methods are used for small-scale refrigeration such as in laboratories and workshops, or in portable coolers.
Ice owes its effectiveness as a cooling agent to its constant melting point of 0 °C (32 °F). In order to melt, ice must absorb 333.1 kJ/kg (143.3 Btu/lb) of heat. Foodstuffs maintained at this temperature or slightly above have an increased storage life. Solid carbon dioxide, known as dry ice, is used also as a refrigerant. Having no liquid phase at normal atmospheric pressure, it sublimes directly from the solid to vapor phase at a temperature of -78.5 °C (-109.3 °F). Dry ice is effective for maintaining products at low temperatures during the period of sublimation.
Cyclic refrigeration
This consists of a refrigeration cycle, where heat is removed from a low-temperature space or source and rejected to a high-temperature sink with the help of external work, and its inverse, the power cycle. In the power cycle, heat is supplied from a high-temperature source to the engine, part of the heat being used to produce work and the rest being rejected to a low-temperature sink. This satisfies the second law of thermodynamics.
A refrigeration cycle describes the changes that take place in the refrigerant as it alternately absorbs and rejects heat as it circulates through a refrigerator. It is also applied to HVACR work, when describing the "process" of refrigerant flow through an HVACR unit, whether it is a packaged or split system.
Heat naturally flows from hot to cold. Work is applied to cool a living space or storage volume by pumping heat from a lower temperature heat source into a higher temperature heat sink. Insulation is used to reduce the work and energy required to achieve and maintain a lower temperature in the cooled space. The operating principle of the refrigeration cycle was described mathematically by Nicolas Léonard Sadi Carnot (1796-1832) in 1824 as a heat engine.
The most common types of refrigeration systems use the reverse-Rankine vapor-compression refrigeration cycle although absorption heat pumps are used in a minority of applications.
Cyclic refrigeration can be classified as:
- Vapour cycle, and
- Gas cycle
Vapour cycle refrigeration can further be classified as:
- Vapour compression refrigeration
- Gas absorption refrigeration
Vapour-compression cycle
- (See Vapour-compression refrigeration for more complete technical details)
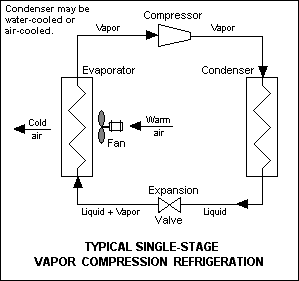
The vapour-compression cycle is used in most household refrigerators as well as in many large commercial and industrial refrigeration systems. Figure 1 provides a schematic diagram of the components of a typical vapour-compression refrigeration system.
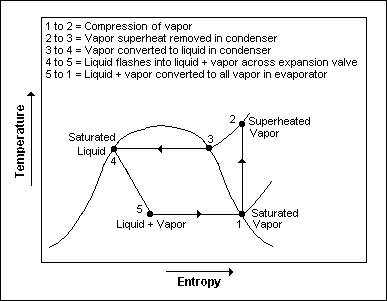
The thermodynamics of the cycle can be analyzed on a diagram[7][8] as shown in Figure 2. In this cycle, a circulating refrigerant such as Freon enters the compressor as a vapour. From point 1 to point 2, the vapour is compressed at constant entropy and exits the compressor superheated. From point 2 to point 3 and on to point 4, the superheated vapour travels through the condenser which first cools and removes the superheat and then condenses the vapour into a liquid by removing additional heat at constant pressure and temperature. Between points 4 and 5, the liquid refrigerant goes through the expansion valve (also called a throttle valve) where its pressure abruptly decreases, causing flash evaporation and auto-refrigeration of, typically, less than half of the liquid.
That results in a mixture of liquid and vapour at a lower temperature and pressure as shown at point 5. The cold liquid-vapour mixture then travels through the evaporator coil or tubes and is completely vaporized by cooling the warm air (from the space being refrigerated) being blown by a fan across the evaporator coil or tubes. The resulting refrigerant vapour returns to the compressor inlet at point 1 to complete the thermodynamic cycle.
The above discussion is based on the ideal vapour-compression refrigeration cycle, and does not take into account real-world effects like frictional pressure drop in the system, slight thermodynamic irreversibility during the compression of the refrigerant vapour, or non-ideal gas behavior (if any).
More information about the design and performance of vapour-compression refrigeration systems is available in the classic "Perry's Chemical Engineers' Handbook".[9]
Vapour absorption cycle
- (See gas absorption refrigerator for more details)
In the early years of the twentieth century, the vapour absorption cycle using water-ammonia systems was popular and widely used but, after the development of the vapour compression cycle, it lost much of its importance because of its low coefficient of performance (about one fifth of that of the vapour compression cycle). Nowadays, the vapour absorption cycle is used only where waste heat is available or where heat is derived from solar collectors.
The absorption cycle is similar to the compression cycle, except for the method of raising the pressure of the refrigerant vapour. In the absorption system, the compressor is replaced by an absorber which dissolves the refrigerant in a suitable liquid, a liquid pump which raises the pressure and a generator which, on heat addition, drives off the refrigerant vapour from the high-pressure liquid. Some work is required by the liquid pump but, for a given quantity of refrigerant, it is much smaller that that needed by the compressor in the vapour compression cycle. In an absorption refrigerator, a suitable combination of refrigerant and absorbent is used. The most common combinations are ammonia (refrigerant) and water (absorbent), and water (refrigerant) and lithium bromide (absorbent).
Gas cycle
When the working fluid is a gas that is compressed and expanded but doesn't change phase, the refrigeration cycle is called a gas cycle. Air is most often this working fluid. As there is no condensation and evaporation intended in a gas cycle, components corresponding to the condenser and evaporator in a vapor compression cycle are the hot and cold gas-to-gas heat exchangers in gas cycles.
The gas cycle is less efficient than the vapor compression cycle because the gas cycle works on the reverse Brayton cycle instead of the reverse Rankine cycle. As such the working fluid does not receive and reject heat at constant temperature. In the gas cycle, the refrigeration effect is equal to the product of the specific heat of the gas and the rise in temperature of the gas in the low temperature side. Therefore, for the same cooling load, a gas refrigeration cycle will require a large mass flow rate and would be bulky.
Because of their lower efficiency and larger bulk, air cycle coolers are not often used nowadays in terrestrial cooling devices. The air cycle machine is very common, however, on gas turbine-powered 'jet' aircraft because compressed air is readily available from the engines' compressor sections. These jet aircrafts' cooling and ventilation units also serve the purpose of pressurizing the aircraft.
Thermoelectric refrigeration
Thermoelectric cooling uses the Peltier effect to create a heat flux between the junction of two different types of materials. This effect is commonly used in camping and portable coolers and for cooling electronic components and small instruments.
Magnetic refrigeration
Magnetic refrigeration, or adiabatic demagnetization, is a cooling technology based on the magnetocaloric effect, an intrinsic property of magnetic solids. The refrigerant is often a paramagnetic salt, such as cerium magnesium nitrate. The active magnetic dipoles in this case are those of the electron shells of the paramagnetic atoms.
A strong magnetic field is applied to the refrigerant, forcing its various magnetic dipoles to align and putting these degrees of freedom of the refrigerant into a state of lowered entropy. A heat sink then absorbs the heat released by the refrigerant due to its loss of entropy. Thermal contact with the heat sink is then broken so that the system is insulated, and the magnetic field is switched off. This increases the heat capacity of the refrigerant, thus decreasing its temperature below the temperature of the heat sink.
Because few materials exhibit the required properties at room temperature, applications have so far been limited to cryogenics and research.
Other methods
Other methods of refrigeration include the Air cycle machine used in aircraft; the Vortex tube used for spot cooling, when compressed air is available; and Thermoacoustic refrigeration using sound waves in a pressurised gas to drive heat transfer and heat exchange.
Unit of refrigeration
Domestic and commercial refrigerators may be rated in kJ/s, or Btu/h of cooling. Commercial refrigerators in the US are mostly rated in tons of refrigeration, but elsewhere in kW. One ton of refrigeration capacity can freeze one short ton of water at 0 °C (32 °F) in 24 hours. Based on that:
- Latent heat of ice (i.e., heat of fusion) ≈ 144 Btu / lb (or 334.5 kJ/kg)
- One short ton = 2000 lb
- Heat to be extracted = 2000 * 144 = 288000 Btu / 24 hours = 12000 Btu/hour = 200 Btu / Minute
- 1 ton refrigeration = 200 Btu / minute = 3.517 kJ/s = 3.517 kilowatts[10]
A much less common definition is: 1 tonne of refrigeration is the rate of heat removal required to freeze a metric ton (i.e., 1000 kg) of water at 0 °C in 24 hours. Based on the heat of fusion being 334.5 kJ/kg, 1 tonne of refrigeration = 13,938 kJ/h = 3.872 kW. As can be seen, 1 tonne of refrigeration is 10% larger than 1 ton of refrigeration.
Most residential air conditioning units range in capacity from about 1 to 5 tons of refrigeration.
See also
- Air conditioning
- Freezer
- Heat pump
- HVAC (Heating, Ventilating and Air-conditioning)
- Icebox
- Ice cream van
- Refrigerant
- Refrigeration cycle
- Refrigerator
- SEER (Seasonal Energy Efficiency Ratio)
- Timeline of low-temperature technology
Notes
- ↑ "Refrigeration fundamentals throughout history"PDF (72.9 KiB)
- ↑ "Air conditioning and refrigeration chronology"PDF (265 KiB)
- ↑ Burstall, Aubrey F. 1965. "A History of Mechanical Engineering". The MIT Press. ISBN 0-262-52001-X.
- ↑ Conditioning and Refrigeration History
- ↑ Monitor Top Refrigerators
- ↑ Molina, Mario J. & Rowland, F. S. 1974. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Department of Chemistry, University of California, Irvine. Nature 249, pp. 810-12.
- ↑ The Ideal Vapor-Compression Cycle
- ↑ Scroll down to "The Basic Vapor Compression Cycle and Components"
- ↑ Perry, R.H. and Green, D.W. 1984. Perry's Chemical Engineers' Handbook. McGraw Hill, Inc. ISBN 0070494797.
- ↑ NIST Guide To SI Units
ReferencesISBN links support NWE through referral fees
- Stoecker, Wilbert F. and Jones, Jerold W. 1983. Refrigeration and Air Conditioning. McGraw Hill Higher Education. ISBN 0070665915.
- Althouse, Andrew D., Carl H. Turnquist, and Alfred F. Bracciano. 2003. Modern Refrigeration and Air Conditioning. Goodheart-Wilcox Publisher; 18 edition. ISBN 1590702808.
- Brain, Marshall. 2006. "How Refrigeration Work". HowStuffWorks.com. Retrieved September 3, 2007.
External links
- American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE)
- British Institute of Refrigeration
- "Notes on vapor-compression refrigeration," Queens University (Canada)
- "The ideal vapor compression refrigeration cycle," University of Nevada (US)
- Refrigeration History
- The Food Refrigeration and Process Engineering Research Centre
- Scroll down to "Continuous-Cycle Absorption System"
- US Department of Energy: Technology Basics of Absorption Cycles
- Refrigeration World
- Calendar of Inventive Contributors to the Development of Refrigeration, 1748-1885, a short history of the evolution of the refrigerator.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.