Difference between revisions of "Nitrous acid" - New World Encyclopedia
({{Paid}}) |
|||
| Line 47: | Line 47: | ||
|- | |- | ||
|} | |} | ||
| − | |||
| − | Nitrous acid is | + | '''Nitrous acid''' (molecular formula [[Hydrogen|H]][[Nitrogen|N]][[Oxygen|O]]<sub>2</sub>) is a weak [[acid]] known only in [[solution]] and in the form of [[nitrite]] salts. In the atmosphere, it is formed as an intermediate, breaking down to produce hydroxyl [[free radical]]s. These free radicals are then involved in regulating the amount of [[ozone]] in the [[troposphere]] (lower atmosphere). |
| + | |||
| + | Nitrous acid is useful for destroying [[sodium azide]] solutions, which are toxic and potentially explosive. It can also be used to prepare [[diazonium salt]]s, which combine with [[aniline]]s and [[phenol]]s to form brightly colored [[azo compound]]s. The latter type of reaction can be used to produce [[azo-dye]]s, as well as serving as a qualitative test for aromatic amines. | ||
==Preparation== | ==Preparation== | ||
| − | |||
| − | == | + | Nitrous acid is readily formed in solution by the action of any mineral acid on [[sodium nitrite]] (NaNO<sub>2</sub>). The reaction may be written as follows: |
| − | + | ::NaNO<sub>2</sub> + H<sup>+</sup> ----> HNO<sub>2</sub> + Na<sup>+</sup> | |
| + | |||
| + | == Chemical reactions == | ||
| + | === Acid-base reactions === | ||
| + | |||
| + | Nitrous acid is a monobasic acid, which means that each molecule of HNO<sub>2</sub> releases one proton (H<sup>+</sup>) in solution. In addition, it is a weak acid, which means that at any given time, the dissociated molecules are in equilibrium with undissociated molecules. | ||
| + | |||
| + | Also, like other acids, nitrous acid reacts with a base to form a salt. In this case, sodium nitrite is formed. | ||
| − | + | ===Decomposition reactions=== | |
| − | + | Nitrous acid is unstable, and it may decompose in two ways, in solution. | |
| − | + | In one type of decomposition, it produces [[nitrogen dioxide]], [[nitric oxide]], and [[water]], as follows: | |
| + | ::2HNO<sub>2</sub> ----> NO<sub>2</sub> + NO + H<sub>2</sub>O | ||
| − | + | In a second pathway, it may decompose to [[nitric acid]], [[nitrous oxide]], and water, as follows: | |
| − | + | ||
| + | ::4HNO<sub>2</sub> ----> 2HNO<sub>3</sub> + N<sub>2</sub>O + H<sub>2</sub>O | ||
==Atmospheric relevance== | ==Atmospheric relevance== | ||
| − | Nitrous acid is an important | + | |
| + | Nitrous acid is formed in the atmosphere as an important intermediate. It is produced by the reaction of nitrogen dioxide (NO<sub>2</sub>) and water on various surfaces such as atmospheric aerosols. The action of sunlight then decomposes it to produce hydroxyl [[free radical]]s, which are intricately involved in regulating the [[ozone]] budget of the troposphere (lower atmosphere). | ||
| + | |||
| + | == Applications == | ||
| + | |||
| + | Nitrous acid is used to destroy toxic and potentially explosive [[sodium azide]] solutions. Nitrous acid can be used to prepare [[diazonium salt]]s, which couple with [[aniline]]s and [[phenol]]s to form brightly colored [[azo compound]]s in a qualitative test for aromatic amines. This reaction is also used to produce [[azo-dye]]s. | ||
==See also== | ==See also== | ||
| − | |||
| − | |||
| − | |||
| − | == | + | * [[Acid]] |
| + | * [[Nitric acid]] | ||
| + | * [[Sulfuric acid]] | ||
| + | |||
| + | == References == | ||
| + | |||
| + | * Chang, Raymond. 2006. ''Chemistry''. 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031 and ISBN 978-0073221038. | ||
| + | |||
| + | * Cotton, F. Albert, Geoffrey Wilkinson, Carlos A. Murillo, and Manfred Bochmann. 1999. ''Advanced Inorganic Chemistry''. 6th edition. New York: Wiley. ISBN 0471199575 | ||
| − | + | * Greenwood, N.N., and A. Earnshaw. 1998. ''Chemistry of the Elements''. 2nd ed. Oxford, UK; Burlington, MA: Butterworth-Heinemann, Elsevier Science. ISBN 0750633654. [http://www.knovel.com/knovel2/Toc.jsp?BookID=402&VerticalID=0 Online version]. | |
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Revision as of 18:35, 27 June 2007
| Nitrous acid | |
|---|---|
| |
| General | |
| Systematic name | Dioxonitric(III) acid |
| Other names | Nitrous acid |
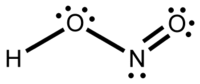
| Molecular formula | HNO2 |
| Molar mass | 47.0134 g/mol |
| CAS number | 7782-77-6 |
| Properties | |
| Density | ? g/cm3 |
| Solubility (water) | |
| Melting point | ? °C |
| Boiling point | ? °C |
| Acid dissociation constant pKa |
3.34 |
| Disclaimer and references | |
Nitrous acid (molecular formula HNO2) is a weak acid known only in solution and in the form of nitrite salts. In the atmosphere, it is formed as an intermediate, breaking down to produce hydroxyl free radicals. These free radicals are then involved in regulating the amount of ozone in the troposphere (lower atmosphere).
Nitrous acid is useful for destroying sodium azide solutions, which are toxic and potentially explosive. It can also be used to prepare diazonium salts, which combine with anilines and phenols to form brightly colored azo compounds. The latter type of reaction can be used to produce azo-dyes, as well as serving as a qualitative test for aromatic amines.
Preparation
Nitrous acid is readily formed in solution by the action of any mineral acid on sodium nitrite (NaNO2). The reaction may be written as follows:
- NaNO2 + H+ ----> HNO2 + Na+
Chemical reactions
Acid-base reactions
Nitrous acid is a monobasic acid, which means that each molecule of HNO2 releases one proton (H+) in solution. In addition, it is a weak acid, which means that at any given time, the dissociated molecules are in equilibrium with undissociated molecules.
Also, like other acids, nitrous acid reacts with a base to form a salt. In this case, sodium nitrite is formed.
Decomposition reactions
Nitrous acid is unstable, and it may decompose in two ways, in solution.
In one type of decomposition, it produces nitrogen dioxide, nitric oxide, and water, as follows:
- 2HNO2 ----> NO2 + NO + H2O
In a second pathway, it may decompose to nitric acid, nitrous oxide, and water, as follows:
- 4HNO2 ----> 2HNO3 + N2O + H2O
Atmospheric relevance
Nitrous acid is formed in the atmosphere as an important intermediate. It is produced by the reaction of nitrogen dioxide (NO2) and water on various surfaces such as atmospheric aerosols. The action of sunlight then decomposes it to produce hydroxyl free radicals, which are intricately involved in regulating the ozone budget of the troposphere (lower atmosphere).
Applications
Nitrous acid is used to destroy toxic and potentially explosive sodium azide solutions. Nitrous acid can be used to prepare diazonium salts, which couple with anilines and phenols to form brightly colored azo compounds in a qualitative test for aromatic amines. This reaction is also used to produce azo-dyes.
See also
ReferencesISBN links support NWE through referral fees
- Chang, Raymond. 2006. Chemistry. 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031 and ISBN 978-0073221038.
- Cotton, F. Albert, Geoffrey Wilkinson, Carlos A. Murillo, and Manfred Bochmann. 1999. Advanced Inorganic Chemistry. 6th edition. New York: Wiley. ISBN 0471199575
- Greenwood, N.N., and A. Earnshaw. 1998. Chemistry of the Elements. 2nd ed. Oxford, UK; Burlington, MA: Butterworth-Heinemann, Elsevier Science. ISBN 0750633654. Online version.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.