Difference between revisions of "Hydrocarbon" - New World Encyclopedia
Rosie Tanabe (talk | contribs) |
|||
| (20 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Copyedited}}{{Paid}}{{Images OK}}{{Submitted}}{{Approved}} | ||
| + | |||
[[Image:MiRO1.jpg|thumb|300px|Hydrocarbons are obtained by refining petroleum at refineries such as this one.]] | [[Image:MiRO1.jpg|thumb|300px|Hydrocarbons are obtained by refining petroleum at refineries such as this one.]] | ||
| − | + | A '''hydrocarbon''' is any [[chemical compound]] that is constituted of just the elements [[carbon]] (C) and [[hydrogen]] (H). Each hydrocarbon molecule consists of a carbon backbone, or "carbon skeleton," with hydrogen [[atom|atoms]] attached to that backbone. | |
| + | {{toc}} | ||
| + | Hydrocarbons are among the [[Earth]]'s most important natural resources. They are currently the main source of the world’s electric [[energy]] and heat energy (such as for heating buildings) because they produce large amounts of heat when burned. The gasoline that serves as fuel for automobiles consists primarily of hydrocarbons. In addition, many hydrocarbons serve as base materials for the synthesis of [[organic chemistry|organic chemicals]] used in the production of consumer products and industrial materials. | ||
| − | + | == Natural occurrence and extraction == | |
| − | + | Hydrocarbons are the main constituents of [[petroleum]] (literally, "rock oil"), also called "oil," and [[natural gas]]. They are commonly found in and extracted from the Earth´s subsurface. Petroleum is a mixture of liquid hydrocarbons, while natural gas is mainly constituted of [[methane]] gas. | |
| − | The | + | The extraction of liquid hydrocarbon [[fuel]] from a number of sedimentary basins has been integral to modern [[energy development]]. Hydrocarbons are [[mining|mined]] from tar sands and oil shale. These reserves require distillation and upgrading to produce synthetic crude and petroleum. A future source of methane may be [[Methane|methane hydrate]]s found on ocean floors. |
| + | |||
| + | == Types of hydrocarbons == | ||
{| align="right" | {| align="right" | ||
|- | |- | ||
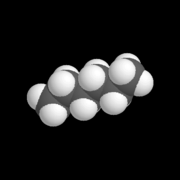
| − | | [[Image: | + | | [[Image:Kalottenmodell_Hexan.png|thumb|right|180px|A model of the alkane known as ''hexane''.]] |
|- | |- | ||
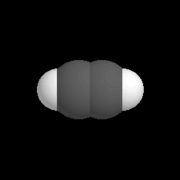
| − | | [[Image: | + | | [[Image:Kalottenmodell_Ethin.png|thumb|right|180px|A model of the alkyne called ''ethyne''.]] |
|- | |- | ||
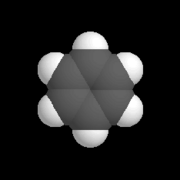
| − | | [[Image: | + | | [[Image:Kalottenmodell_Benzol.png|thumb|right|180px|A model of an arene called ''benzene''.]] |
|} | |} | ||
| − | == | + | There are essentially three types of hydrocarbons: |
| + | Saturated hydrocarbons, also known as [[alkane]]s: In each molecule of an alkane, the [[chemical bond]]s that join the carbon atoms are single [[covalent bond]]s. If the alkane molecule includes a ring of carbon atoms (all connected by single covalent bonds), it is called a cycloalkane. | ||
| + | Unsaturated hydrocarbons, which are subdivided into two groups: | ||
| + | #*[[alkenes]]: Each molecule of an alkene contains at least one double covalent bond between carbon atoms. | ||
| + | #*[[alkynes]]: Each molecule of an alkyne contains at least one triple covalent bond between carbon atoms. | ||
| + | #[[Aromatic hydrocarbon]]s, or [[arenes]]: Each molecule of an aromatic hydrocarbon contains at least one [[aromatic ring]], in which the bonds between carbon atoms are [[aromatic bond]]s. | ||
| + | |||
| + | When organic compounds are considered in general, saturated and unsaturated hydrocarbons are placed in the category known as ''aliphatic compounds'', while aromatic hydrocarbons are categorized as ''aromatic compounds''. | ||
| + | |||
| + | === Some simple hydrocarbons === | ||
| + | |||
| + | The simplest hydrocarbon is [[methane]], the main constituent of [[natural gas]]. Its chemical formula, CH<sub>4</sub>, indicates that each molecule of methane consists of four hydrogen atoms attached to one carbon atom. [[Ethane]], with the formula C<sub>2</sub>H<sub>6</sub>, is a hydrocarbon (more specifically, an [[alkane]]) in which each molecule has two carbon atoms held together with a single [[covalent bond]], and three hydrogen atoms are bound to each carbon atom. Each molecule of [[propane]] (C<sub>3</sub>H<sub>8</sub>) has three carbon atoms, and each molecule of [[butane]] (C<sub>4</sub>H<sub>10</sub>) has four carbons. | ||
| − | + | === General chemical formulas === | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | If a hydrocarbon molecule contains "n" carbon atoms, and the type of hydrocarbon is known, its general chemical formula can be written as follows: | |
| + | *Alkane: C<sub>''n''</sub>H<sub>''2n+2''</sub>. | ||
| + | *Cycloalkane: C<sub>''n''</sub>H<sub>''2n''</sub> (assuming a single ring, with all the carbon atoms in the ring structure). | ||
| + | *Alkene: C<sub>''n''</sub>H<sub>''2n''</sub> (assuming only one double bond in each molecule). | ||
| + | *Alkyne: C<sub>''n''</sub>H<sub>''2n-2''</sub> (assuming only one triple bond in each molecule). | ||
| + | *Aromatic ring: C<sub>''n''</sub>H<sub>''n''</sub> (assuming a single ring, with all the carbon atoms in the ring structure). | ||
| − | The | + | The above chemical formulas are based on the assumption that each carbon atom forms four covalent bonds, including bonds with hydrogen atoms and other carbon atoms. A double covalent bond counts as two bonds; a triple covalent bond counts as three bonds. A carbon atom in an aromatic ring is a special case and can have only one hydrogen atom attached to it. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == Molecular graph== | + | === Molecular graph === |
| − | |||
| − | + | A "molecular graph" of a hydrocarbon is a simple representation of the carbon skeleton of the molecule under consideration. Each line in the molecular graph represents a covalent bond that connects two carbon atoms. Thus, although the graph shows no symbols for carbon atoms, a carbon atom is (by convention) located at each end of each line. The symbols for hydrogen atoms are not shown either, but each carbon atom holds as many hydrogen atoms as it needs to form four covalent bonds. | |
| − | |||
| − | + | ==Uses of hydrocarbons== | |
| − | + | Most hydrocarbons are combustible. When burned, they produce carbon dioxide, water, and large quantities of heat. This energy is used to heat homes and other buildings and to generate electricity. When heating a home, for example, oil or natural gas is burned and the energy released is used to heat water or air. The hot water or air is then circulated around the building. | |
| − | + | A similar principle is used to create electric energy in [[power plants]]. A hydrocarbon fuel (such as natural gas) is burned, the energy released is used to convert water into [[steam]], and the steam is used to drive [[turbine]]s that generate electricity. | |
| − | + | Hydrocarbons are also raw materials that serve as [[feedstock]] for the production of a wide range of organic chemicals, which in turn are used for such products as plastics, pigments, solvents, pharmaceuticals, and explosives. | |
| − | + | == Effects on health and the environment == | |
| − | + | Hydrocarbon vapors can be harmful if inhaled. Moreover, hydrocarbons contribute to the formation of [[ozone]] in the [[Earth's atmosphere|troposphere]]. For these reasons, hydrocarbons in the atmosphere are considered to be pollutants. | |
| − | + | Ideally, the combustion of hydrocarbons should produce only carbon dioxide, water, and heat. Yet, incomplete combustion leads to the production of carbon monoxide, a toxic gas. Carbon monoxide binds to [[hemoglobin]] (in the blood) more readily than oxygen does; so, when carbon monoxide is inhaled, it blocks oxygen from being absorbed and leads to suffocation. Moreover, the hydrocarbon fuel being burned may contain other substances that are harmful when released. | |
| − | + | Moreover, carbon dioxide, a product of the combustion of hydrocarbons, is a greenhouse gas—a gas that helps trap heat in the [[Earth's atmosphere]]. This has led to concerns that the excessive burning of hydrocarbon fuels contributes to [[global warming]]. | |
| − | |||
| − | |||
| − | |||
== See also == | == See also == | ||
| − | *[[ | + | |
| − | *[[ | + | *[[Alkane]] |
| − | *[[ | + | *[[Alkene]] |
| − | *[[ | + | *[[Alkyne]] |
| − | *[[ | + | *[[Aromatic compound]] |
| + | *[[Natural gas]] | ||
| + | *[[Petroleum]] | ||
| + | |||
| + | == References == | ||
| + | |||
| + | * McMurry, John. 2004. ''Organic Chemistry,'' 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052. | ||
| + | |||
| + | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry,'' 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0136436692. | ||
| + | |||
| + | * Solomons, T.W. Graham, and Craig B. Fryhle 2004. ''Organic Chemistry,'' 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998. | ||
| + | |||
| + | == External links == | ||
| + | All links retrieved May 17, 2021. | ||
| + | * [http://www.worldofmolecules.com/fuels/methane.htm The Methane Molecule] | ||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
| − | + | ||
{{credit|65832974}} | {{credit|65832974}} | ||
Latest revision as of 13:34, 17 May 2021
A hydrocarbon is any chemical compound that is constituted of just the elements carbon (C) and hydrogen (H). Each hydrocarbon molecule consists of a carbon backbone, or "carbon skeleton," with hydrogen atoms attached to that backbone.
Hydrocarbons are among the Earth's most important natural resources. They are currently the main source of the world’s electric energy and heat energy (such as for heating buildings) because they produce large amounts of heat when burned. The gasoline that serves as fuel for automobiles consists primarily of hydrocarbons. In addition, many hydrocarbons serve as base materials for the synthesis of organic chemicals used in the production of consumer products and industrial materials.
Natural occurrence and extraction
Hydrocarbons are the main constituents of petroleum (literally, "rock oil"), also called "oil," and natural gas. They are commonly found in and extracted from the Earth´s subsurface. Petroleum is a mixture of liquid hydrocarbons, while natural gas is mainly constituted of methane gas.
The extraction of liquid hydrocarbon fuel from a number of sedimentary basins has been integral to modern energy development. Hydrocarbons are mined from tar sands and oil shale. These reserves require distillation and upgrading to produce synthetic crude and petroleum. A future source of methane may be methane hydrates found on ocean floors.
Types of hydrocarbons
There are essentially three types of hydrocarbons: Saturated hydrocarbons, also known as alkanes: In each molecule of an alkane, the chemical bonds that join the carbon atoms are single covalent bonds. If the alkane molecule includes a ring of carbon atoms (all connected by single covalent bonds), it is called a cycloalkane. Unsaturated hydrocarbons, which are subdivided into two groups:
- alkenes: Each molecule of an alkene contains at least one double covalent bond between carbon atoms.
- alkynes: Each molecule of an alkyne contains at least one triple covalent bond between carbon atoms.
- Aromatic hydrocarbons, or arenes: Each molecule of an aromatic hydrocarbon contains at least one aromatic ring, in which the bonds between carbon atoms are aromatic bonds.
When organic compounds are considered in general, saturated and unsaturated hydrocarbons are placed in the category known as aliphatic compounds, while aromatic hydrocarbons are categorized as aromatic compounds.
Some simple hydrocarbons
The simplest hydrocarbon is methane, the main constituent of natural gas. Its chemical formula, CH4, indicates that each molecule of methane consists of four hydrogen atoms attached to one carbon atom. Ethane, with the formula C2H6, is a hydrocarbon (more specifically, an alkane) in which each molecule has two carbon atoms held together with a single covalent bond, and three hydrogen atoms are bound to each carbon atom. Each molecule of propane (C3H8) has three carbon atoms, and each molecule of butane (C4H10) has four carbons.
General chemical formulas
If a hydrocarbon molecule contains "n" carbon atoms, and the type of hydrocarbon is known, its general chemical formula can be written as follows:
- Alkane: CnH2n+2.
- Cycloalkane: CnH2n (assuming a single ring, with all the carbon atoms in the ring structure).
- Alkene: CnH2n (assuming only one double bond in each molecule).
- Alkyne: CnH2n-2 (assuming only one triple bond in each molecule).
- Aromatic ring: CnHn (assuming a single ring, with all the carbon atoms in the ring structure).
The above chemical formulas are based on the assumption that each carbon atom forms four covalent bonds, including bonds with hydrogen atoms and other carbon atoms. A double covalent bond counts as two bonds; a triple covalent bond counts as three bonds. A carbon atom in an aromatic ring is a special case and can have only one hydrogen atom attached to it.
Molecular graph
A "molecular graph" of a hydrocarbon is a simple representation of the carbon skeleton of the molecule under consideration. Each line in the molecular graph represents a covalent bond that connects two carbon atoms. Thus, although the graph shows no symbols for carbon atoms, a carbon atom is (by convention) located at each end of each line. The symbols for hydrogen atoms are not shown either, but each carbon atom holds as many hydrogen atoms as it needs to form four covalent bonds.
Uses of hydrocarbons
Most hydrocarbons are combustible. When burned, they produce carbon dioxide, water, and large quantities of heat. This energy is used to heat homes and other buildings and to generate electricity. When heating a home, for example, oil or natural gas is burned and the energy released is used to heat water or air. The hot water or air is then circulated around the building.
A similar principle is used to create electric energy in power plants. A hydrocarbon fuel (such as natural gas) is burned, the energy released is used to convert water into steam, and the steam is used to drive turbines that generate electricity.
Hydrocarbons are also raw materials that serve as feedstock for the production of a wide range of organic chemicals, which in turn are used for such products as plastics, pigments, solvents, pharmaceuticals, and explosives.
Effects on health and the environment
Hydrocarbon vapors can be harmful if inhaled. Moreover, hydrocarbons contribute to the formation of ozone in the troposphere. For these reasons, hydrocarbons in the atmosphere are considered to be pollutants.
Ideally, the combustion of hydrocarbons should produce only carbon dioxide, water, and heat. Yet, incomplete combustion leads to the production of carbon monoxide, a toxic gas. Carbon monoxide binds to hemoglobin (in the blood) more readily than oxygen does; so, when carbon monoxide is inhaled, it blocks oxygen from being absorbed and leads to suffocation. Moreover, the hydrocarbon fuel being burned may contain other substances that are harmful when released.
Moreover, carbon dioxide, a product of the combustion of hydrocarbons, is a greenhouse gas—a gas that helps trap heat in the Earth's atmosphere. This has led to concerns that the excessive burning of hydrocarbon fuels contributes to global warming.
See also
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0136436692.
- Solomons, T.W. Graham, and Craig B. Fryhle 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
External links
All links retrieved May 17, 2021.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.