Difference between revisions of "Catecholamine" - New World Encyclopedia
Rick Swarts (talk | contribs) |
m (Robot: Remove contracted tag) |
||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Copyedited}}{{Approved}}{{Images OK}}{{Submitted}}{{Paid}} |
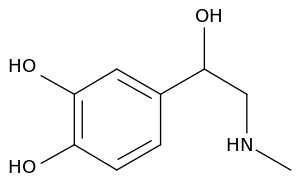
[[Image:Adrenaline.svg|thumb|[[epinephrine]]]] | [[Image:Adrenaline.svg|thumb|[[epinephrine]]]] | ||
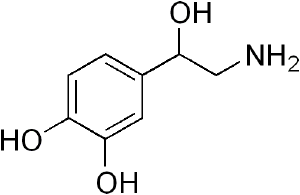
[[Image:Norepinephrine structure.png|thumb|[[norepinephrine]]]] | [[Image:Norepinephrine structure.png|thumb|[[norepinephrine]]]] | ||
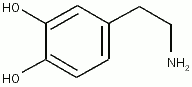
[[Image:Dopamine.png|thumb|[[dopamine]]]] | [[Image:Dopamine.png|thumb|[[dopamine]]]] | ||
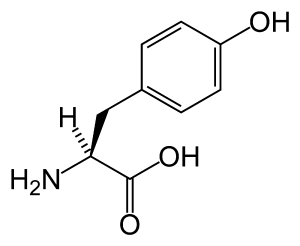
| − | [[Image:L-tyrosine-skeletal.png|thumb|[[tyrosine]] is the precursor of catecholamines]] | + | [[Image:L-tyrosine-skeletal.png|thumb|[[tyrosine]] is the precursor of catecholamines.]] |
| − | '''Catecholamine''' is any of a group of | + | '''Catecholamine''' is any of a group of [[amine]]s (nitrogen-contain organic compounds) derived from the [[amino acid]] [[tyrosine]] and containing a [[catechol]] group (aromatic chemical compound consisting of a benzene ring with two hydroxyl groups). Catecholamine are important as [[neurotransmitter]]s and [[hormone]]s. The most abundant catecholamines are [[epinephrine]] (adrenaline), [[norepinephrine]] (noradrenaline) and [[dopamine]], all of which are produced by phenylalanine and tyrosine. |
| − | Catecholamines as [[hormone]] are released by the [[adrenal gland]]s in situations of [[stress (medical)|stress] such as | + | Catecholamines as [[hormone]] are released by the [[adrenal gland]]s in situations of [[stress (medical)|stress]] such as psychological stress or [[low blood sugar]] levels (Hoffman 1999). |
| + | |||
| + | The synthesis, function, and degradation of catecholamines reflects the complexity and harmonious coordination in bodily systems. Their synthesis and degradation involve a series of [[enzyme|enzymatic]] steps, and as hormones the catecholamine's epinephrine, norepinehprine, and dopamine are produced in one part of the body only to impact cells in other parts. Various [[#Disorders|disorders]] result when this complex coordination is disrupted. | ||
==Structure== | ==Structure== | ||
| − | Catecholamines are [[chemical | + | Catecholamines are [[chemical compound]]s that are derived from [[tyrosine]] and contain both a catechol group and an amine group. |
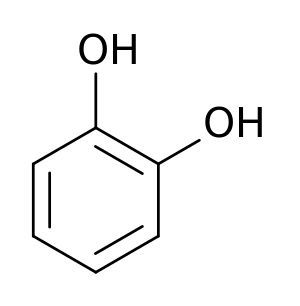
[[Image:Pyrocatechol.svg|thumb|left|Catechol]] | [[Image:Pyrocatechol.svg|thumb|left|Catechol]] | ||
| − | Tyrosine is an α-[[amino acid]] that is found in most [[protein]]s and is normally readily converted from the essential amino acid [[phenylalanine]] in the [[human body]]. Tyrosine's chemical formula is C<sub>9</sub>H<sub>11</sub>NO<sub>3</sub> (IUPAC-IUB 1983) (that is, one more [[nitrogen]] atom than phenylalanine) and it contains a large rigid aromatic group on the side chain—a [[phenol]] side chain with a [[hydroxyl]] group. Catechol or pyrocatechol is a [[benzenediol]] (aromatic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring) with the formula C<sub>6</sub>H<sub>4</sub>(OH)<sub>2</sub>. An amine group is a functional group that | + | Tyrosine is an α-[[amino acid]] that is found in most [[protein]]s and is normally readily converted from the essential amino acid [[phenylalanine]] in the [[human body]]. Tyrosine's chemical formula is C<sub>9</sub>H<sub>11</sub>NO<sub>3</sub> (IUPAC-IUB 1983) (that is, one more [[nitrogen]] atom than phenylalanine) and it contains a large rigid aromatic group on the side chain—a [[phenol]] side chain with a [[hydroxyl]] group. Catechol or pyrocatechol is a [[benzenediol]] (aromatic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring) with the formula C<sub>6</sub>H<sub>4</sub>(OH)<sub>2</sub>. An amine group is a functional group that contains nitrogen as the key atom. Thus, a catecolamine has the distinct structure of a [[benzene ring]] with two hydroxyl groups, an intermediate [[ethyl]] chain, and a terminal [[amine]] group. Some of them are [[biogenic amine]]s (substances produced by life processes). |
| − | Catecholamines are water soluble and are 50 | + | Catecholamines are [[water]] soluble and are 50 percent bound to plasma proteins, so they circulate in the [[blood]]stream. |
==Production== | ==Production== | ||
| − | + | Catecholamines are produced mainly by the [[chromaffin cells]] of the [[adrenal gland|adrenal medulla]] and the [[postganglionic fiber]]s of the [[autonomic nervous system|sympathetic nervous system]]. Epinephrine (or [[adrenaline]]) and norepinephrine ([[noradrenaline]]) are [[hormone]]s that are secreted principally by the adrenal medulla. The adrenal gland, located atop the [[kidney]]s, is separated into two distinct structures, the adrenal medulla and the adrenal cortex. The adrenal medulla is at the center of the adrenal gland and is surrounded by the adrenal cortex, with the adrenal medulla taking up about one-quarter of the adrenal gland and the adrenal cortex the remaining three-quarters. The adrenal glands are chiefly responsible for regulating the [[stress (medicine)|stress]] response through the synthesis of [[corticosteroid]]s and catecholamines. | |
| − | Catecholamines are produced mainly by the [[chromaffin cells]] of the [[adrenal gland|adrenal medulla]] and the [[postganglionic fiber]]s of the [[autonomic nervous system|sympathetic nervous system]]. Epinephrine (or [[adrenaline]]) and norepinephrine ([[noradrenaline]]) are [[hormone]]s that are secreted principally by the adrenal medulla. The | ||
[[Dopamine]], which acts as a [[neurotransmitter]] in the [[central nervous system]], is largely produced in neuronal cell bodies in two areas of the brainstem: the [[substantia nigra]] and the [[ventral tegmental area]]. | [[Dopamine]], which acts as a [[neurotransmitter]] in the [[central nervous system]], is largely produced in neuronal cell bodies in two areas of the brainstem: the [[substantia nigra]] and the [[ventral tegmental area]]. | ||
| − | Tryosine, one of the main precursors of | + | [[Image:Biosynthese Adrenalin.png|thumb|350px|Epinephrine is synthesized from [[norepinephrine]] in a synthetic pathway shared by all catecholamines.]] |
| − | + | There is a basic synthetic pathway shared by all catecholamines. [[Tryosine]], one of the main precursors of catecholamines, is created from [[phenylalanine]] by hydroxylation via the [[enzyme]] phenylalanine hydroxylase. (Tyrosine is also ingested directly from dietary protein). Tyrosine is then converted to [[L-dopa]]. Further reactions convert it to [[dopamine]], and eventually to norepinephrine and epinephrine (Joh and Hwang 1987). | |
| − | + | For example, [[norepinephrine]] (noradrenaline) is synthesized by a series of enzymatic steps in the adrenal medulla according to the following process: | |
| − | * The first reaction is the oxidation into dihydroxyphenylalanine (L-DOPA). | + | * The first reaction is the oxidation of tyrosine into dihydroxyphenylalanine (L-DOPA). |
* This is followed by decarboxylation into the neurotransmitter dopamine. | * This is followed by decarboxylation into the neurotransmitter dopamine. | ||
* Last is the final β-oxidation into norepinephrine by dopamine beta hydroxylase. | * Last is the final β-oxidation into norepinephrine by dopamine beta hydroxylase. | ||
| − | + | Epinephrine (adrenaline) then is synthesized from norepinephrine in this synthetic pathway involving L-dopa, dopamine, norepinephrine, and epinephrine. Epinephrine is synthesized via methylation of the primary distal amine of norepinephrine by phenylethanolamine N-methyltransferase (PNMT) in the [[cytoplasm|cytosol]] of adrenergic neurons and cells of the adrenal medulla (so-called chromaffin cells). PNMT is only found in the cytosol of cells of adrenal medullary cells. PNMT uses ''S''-adenosylmethionine (SAMe) as a cofactor to donate the [[methyl]] group to norepinephrine, creating epinephrine. | |
| − | Epinephrine is synthesized from | ||
| − | |||
| − | Epinephrine is synthesized via methylation of the primary distal amine of norepinephrine by phenylethanolamine N-methyltransferase (PNMT) in the [[cytoplasm|cytosol]] of adrenergic neurons and cells of the | ||
For norepinephrine to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H<sup>+</sup> exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized epinephrine from the cytosol back into chromaffin granules in preparation for release. | For norepinephrine to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H<sup>+</sup> exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized epinephrine from the cytosol back into chromaffin granules in preparation for release. | ||
| − | |||
==Function== | ==Function== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | Two catecholamines, [[norepinephrine]] (noradrenaline) and [[dopamine]], act as [[neurotransmitter]]s in the [[central nervous system]] and as [[hormone]]s in the blood circulation. The catecholamine norepinephrine is a neurotransmitter of the [[autonomic nervous system|sympathetic nervous system]] but is also present in the blood (mostly through "spillover" from the [[synapse]]s of the sympathetic system). [[Epinephrine]] (adrenaline) is a hormone that is secreted principally in response to physical or mental [[stress (medicine)|stress]]. | |
| − | + | As hormones, the catecholamines epinephrine and norepinephrine underlies the fight-or-flight response to psychological or environmental stressors such as [[noise health effects|elevated sound levels]], [[light pollution|intense light]], or [[Hypoglycemia|low blood sugar levels]]. Some typical actions of these catecholamines acting as hormones include increasing [[heart]] rate and blood pressure, triggering the release of [[glucose]] from energy stores, and increasing skeletal [[muscle]] readiness, among other sympathetic nervous system actions. | |
| − | |||
| + | Some drugs, like [[tolcapone]] (a central [[COMT]]-inhibitor), raise the levels of all the catecholamines. | ||
==Degradation== | ==Degradation== | ||
| − | + | Catecholamines have a half-life of approximately a few minutes when circulating in the [[blood]]. | |
Monoamine oxidase ([[MAO]]) is the main enzyme responsible for degradation of catecholamines. | Monoamine oxidase ([[MAO]]) is the main enzyme responsible for degradation of catecholamines. | ||
| Line 57: | Line 50: | ||
[[Methamphetamine]] and [[MAOI]]s bind to MAOs to inhibit their action of breaking down catecholamines. This is primarily the reason why the effects of amphetamines last longer than [[cocaine]] and other substances. Amphetamines not only causes a release of [[dopamine]], [[epinephrine]], and [[norepinephrine]] into the blood stream, but also keeps it working there for a long time. | [[Methamphetamine]] and [[MAOI]]s bind to MAOs to inhibit their action of breaking down catecholamines. This is primarily the reason why the effects of amphetamines last longer than [[cocaine]] and other substances. Amphetamines not only causes a release of [[dopamine]], [[epinephrine]], and [[norepinephrine]] into the blood stream, but also keeps it working there for a long time. | ||
| − | == | + | ==Disorders== |
| − | + | Extremely high levels of catecholamine (also known as catecholamine toxicity) can occur in [[Central nervous system|CNS]] trauma due to stimulation and/or damage of [[nucleus (neuroanatomy)|nuclei]] in the [[brainstem]], particularly those nuclei affecting the [[sympathetic nervous system]]. In emergency medicine, this occurrence is widely known as ''catecholamine dump''. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | There are a number of specific disorders involving catecholamines, including neuroblastoma, pheochromocytoma, chemodectoma, familial paraganglioma syndrome, dopamine-β-hydroxalase deficiency, and tetrahydrobiopterin deficiency (MN 2007). | |
| + | ''Neuroblastoma''. Neuroblastoma is the most common extracranial solid [[cancer]] in infancy and childhood. It is a neuroendocrine tumor, arising from any neural crest element of the [[sympathetic nervous system]]. Essentially, neuroblastoma is a cancer of the sympathetic nervous system—a nerve network that carries messages from the brain throughout the body. Its solid tumors, which take the form of a lump or mass, commonly begin in one of the [[adrenal gland]]s, though they can also develop in nerve tissues in the neck, chest, abdomen, or pelvis. Neuroblastoma usually produces catecholamines (MN 2007). | ||
| − | + | ''Pheochromocytoma''. Pheochromocytoma is a [[neuroendocrine tumor]] of the [[Adrenal gland|medulla]] of the adrenal glands originating in the [[chromaffin]] cells, which secretes excessive amounts of catecholamines, usually [[adrenaline]] and [[noradrenaline]]. Symptoms include hypertension, sweating, nausea and vomiting, headache, pallor, and severe apprehension (MN 2007). | |
| − | + | ''Chemodectoma''. Chemodectoma, also known as nonchromaffin paraganglioma, is a generally benign (sometimes malignant) tumor of the chemoreceptor system. This rare [[neoplasm]] can be found in the abdomen, thorax, and in the head and neck region. | |
| − | + | ''Familial paraganglioma syndrome''. Familial paraganglioma syndrome is an unusual familial disease involving tumors (paragangliomas, glomus tumors, or chemodectomas) that are slow growing and normally in the head and neck region (MN 2007). | |
| − | |||
| − | |||
| + | ==References== | ||
| − | == | + | * Hoffman, R. 1999. [http://www.consciouschoice.com/1999/cc1207/hmd1207.html Hypoglycemia] ''Conscious Choice: The Holistic M.D.''. Retrieved October 23, 2007. |
| − | * | + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology] ''IUPAC-IUB''. Retrieved October 23, 2007. |
| + | * Joh, T. H., and O. Hwang. 1987. [http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=3473965&dopt=Abstract Dopamine beta-hydroxylase: Biochemistry and molecular biology] ''Ann N Y Acad Sci.'' 493: 342-50. Retrieved October 23, 2007. | ||
| + | * MedicineNet. 2007. [http://www.medterms.com/script/main/art.asp?articlekey=20210 Definition of catecholamine] ''MedicineNet.com''. Retrieved October 23, 2007. | ||
| − | [[Category:Life sciences]] | + | [[Category:Life sciences]][[Category:Biochemistry]] |
| − | {{credit|143561840}} | + | {{credit|Catecholamine|143561840|Neuroblastoma|148691123|Pheochromocytoma|147739015|Paraganglioma|144004369}} |
Latest revision as of 18:59, 3 April 2008
Catecholamine is any of a group of amines (nitrogen-contain organic compounds) derived from the amino acid tyrosine and containing a catechol group (aromatic chemical compound consisting of a benzene ring with two hydroxyl groups). Catecholamine are important as neurotransmitters and hormones. The most abundant catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline) and dopamine, all of which are produced by phenylalanine and tyrosine.
Catecholamines as hormone are released by the adrenal glands in situations of stress such as psychological stress or low blood sugar levels (Hoffman 1999).
The synthesis, function, and degradation of catecholamines reflects the complexity and harmonious coordination in bodily systems. Their synthesis and degradation involve a series of enzymatic steps, and as hormones the catecholamine's epinephrine, norepinehprine, and dopamine are produced in one part of the body only to impact cells in other parts. Various disorders result when this complex coordination is disrupted.
Structure
Catecholamines are chemical compounds that are derived from tyrosine and contain both a catechol group and an amine group.
Tyrosine is an α-amino acid that is found in most proteins and is normally readily converted from the essential amino acid phenylalanine in the human body. Tyrosine's chemical formula is C9H11NO3 (IUPAC-IUB 1983) (that is, one more nitrogen atom than phenylalanine) and it contains a large rigid aromatic group on the side chain—a phenol side chain with a hydroxyl group. Catechol or pyrocatechol is a benzenediol (aromatic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring) with the formula C6H4(OH)2. An amine group is a functional group that contains nitrogen as the key atom. Thus, a catecolamine has the distinct structure of a benzene ring with two hydroxyl groups, an intermediate ethyl chain, and a terminal amine group. Some of them are biogenic amines (substances produced by life processes).
Catecholamines are water soluble and are 50 percent bound to plasma proteins, so they circulate in the bloodstream.
Production
Catecholamines are produced mainly by the chromaffin cells of the adrenal medulla and the postganglionic fibers of the sympathetic nervous system. Epinephrine (or adrenaline) and norepinephrine (noradrenaline) are hormones that are secreted principally by the adrenal medulla. The adrenal gland, located atop the kidneys, is separated into two distinct structures, the adrenal medulla and the adrenal cortex. The adrenal medulla is at the center of the adrenal gland and is surrounded by the adrenal cortex, with the adrenal medulla taking up about one-quarter of the adrenal gland and the adrenal cortex the remaining three-quarters. The adrenal glands are chiefly responsible for regulating the stress response through the synthesis of corticosteroids and catecholamines.
Dopamine, which acts as a neurotransmitter in the central nervous system, is largely produced in neuronal cell bodies in two areas of the brainstem: the substantia nigra and the ventral tegmental area.
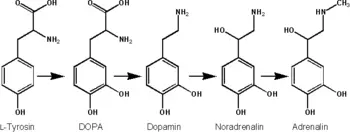
There is a basic synthetic pathway shared by all catecholamines. Tryosine, one of the main precursors of catecholamines, is created from phenylalanine by hydroxylation via the enzyme phenylalanine hydroxylase. (Tyrosine is also ingested directly from dietary protein). Tyrosine is then converted to L-dopa. Further reactions convert it to dopamine, and eventually to norepinephrine and epinephrine (Joh and Hwang 1987).
For example, norepinephrine (noradrenaline) is synthesized by a series of enzymatic steps in the adrenal medulla according to the following process:
- The first reaction is the oxidation of tyrosine into dihydroxyphenylalanine (L-DOPA).
- This is followed by decarboxylation into the neurotransmitter dopamine.
- Last is the final β-oxidation into norepinephrine by dopamine beta hydroxylase.
Epinephrine (adrenaline) then is synthesized from norepinephrine in this synthetic pathway involving L-dopa, dopamine, norepinephrine, and epinephrine. Epinephrine is synthesized via methylation of the primary distal amine of norepinephrine by phenylethanolamine N-methyltransferase (PNMT) in the cytosol of adrenergic neurons and cells of the adrenal medulla (so-called chromaffin cells). PNMT is only found in the cytosol of cells of adrenal medullary cells. PNMT uses S-adenosylmethionine (SAMe) as a cofactor to donate the methyl group to norepinephrine, creating epinephrine.
For norepinephrine to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H+ exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized epinephrine from the cytosol back into chromaffin granules in preparation for release.
Function
Two catecholamines, norepinephrine (noradrenaline) and dopamine, act as neurotransmitters in the central nervous system and as hormones in the blood circulation. The catecholamine norepinephrine is a neurotransmitter of the sympathetic nervous system but is also present in the blood (mostly through "spillover" from the synapses of the sympathetic system). Epinephrine (adrenaline) is a hormone that is secreted principally in response to physical or mental stress.
As hormones, the catecholamines epinephrine and norepinephrine underlies the fight-or-flight response to psychological or environmental stressors such as elevated sound levels, intense light, or low blood sugar levels. Some typical actions of these catecholamines acting as hormones include increasing heart rate and blood pressure, triggering the release of glucose from energy stores, and increasing skeletal muscle readiness, among other sympathetic nervous system actions.
Some drugs, like tolcapone (a central COMT-inhibitor), raise the levels of all the catecholamines.
Degradation
Catecholamines have a half-life of approximately a few minutes when circulating in the blood.
Monoamine oxidase (MAO) is the main enzyme responsible for degradation of catecholamines.
Methamphetamine and MAOIs bind to MAOs to inhibit their action of breaking down catecholamines. This is primarily the reason why the effects of amphetamines last longer than cocaine and other substances. Amphetamines not only causes a release of dopamine, epinephrine, and norepinephrine into the blood stream, but also keeps it working there for a long time.
Disorders
Extremely high levels of catecholamine (also known as catecholamine toxicity) can occur in CNS trauma due to stimulation and/or damage of nuclei in the brainstem, particularly those nuclei affecting the sympathetic nervous system. In emergency medicine, this occurrence is widely known as catecholamine dump.
There are a number of specific disorders involving catecholamines, including neuroblastoma, pheochromocytoma, chemodectoma, familial paraganglioma syndrome, dopamine-β-hydroxalase deficiency, and tetrahydrobiopterin deficiency (MN 2007).
Neuroblastoma. Neuroblastoma is the most common extracranial solid cancer in infancy and childhood. It is a neuroendocrine tumor, arising from any neural crest element of the sympathetic nervous system. Essentially, neuroblastoma is a cancer of the sympathetic nervous system—a nerve network that carries messages from the brain throughout the body. Its solid tumors, which take the form of a lump or mass, commonly begin in one of the adrenal glands, though they can also develop in nerve tissues in the neck, chest, abdomen, or pelvis. Neuroblastoma usually produces catecholamines (MN 2007).
Pheochromocytoma. Pheochromocytoma is a neuroendocrine tumor of the medulla of the adrenal glands originating in the chromaffin cells, which secretes excessive amounts of catecholamines, usually adrenaline and noradrenaline. Symptoms include hypertension, sweating, nausea and vomiting, headache, pallor, and severe apprehension (MN 2007).
Chemodectoma. Chemodectoma, also known as nonchromaffin paraganglioma, is a generally benign (sometimes malignant) tumor of the chemoreceptor system. This rare neoplasm can be found in the abdomen, thorax, and in the head and neck region.
Familial paraganglioma syndrome. Familial paraganglioma syndrome is an unusual familial disease involving tumors (paragangliomas, glomus tumors, or chemodectomas) that are slow growing and normally in the head and neck region (MN 2007).
ReferencesISBN links support NWE through referral fees
- Hoffman, R. 1999. Hypoglycemia Conscious Choice: The Holistic M.D.. Retrieved October 23, 2007.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB. Retrieved October 23, 2007.
- Joh, T. H., and O. Hwang. 1987. Dopamine beta-hydroxylase: Biochemistry and molecular biology Ann N Y Acad Sci. 493: 342-50. Retrieved October 23, 2007.
- MedicineNet. 2007. Definition of catecholamine MedicineNet.com. Retrieved October 23, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.