Aspartic acid
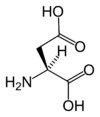 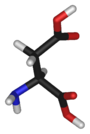 Chemical structure of L-aspartic acid | |
Aspartic acid | |
| Systematic (IUPAC) name | |
| (2S)-2-aminobutanedioic acid | |
| Identifiers | |
| CAS number | 56-84-8 |
| PubChem | 5960 |
| Chemical data | |
| Formula | C4H7NO4 |
| Mol. weight | 133.10 |
| SMILES | N[C@@H](CC(O)=O)C(O)=O |
| Complete data | |
Aspartic acid is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CO2H. The L-isomer is one of the 20 proteinogenic amino acids, i.e. the building blocks of proteins. Its three letter code is asp, its one letter code is D, and its codons are GAU and GAC.[1] It is classified as an acidic amino acid, together with glutamic acid. Aspartic acid is pervasive in biosynthesis.
Note: Alanine is an one of the simplest amino acids in terms of molecular structure and one of the most widely found in protein. In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids required for normal functioning. However, it is considered to be non-essential since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. It has the chemical formula HO2CCH(NH2)CH3.
Alanine's three letter code is ala, its one letter code is A, and its codons are GCU, GCC, GCA, and GCG (IUPAC-IUB 1983).
Aspartic acid Aspd D d 2-Aminobutanedioic acid HOOC-CH2-CH(NH2)-COOH systematic name
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid. The exception to this basic structure is proline, whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis.
Role in biosynthesis of amino acids
Aspartic acid is non-essential in mammals, being produced from oxaloacetate by transamination. In plants and microorganisms, aspartic acid is the precursor to several amino acids, including four that are essential: methionine, threonine, isoleucine, and lysine. The conversion of aspartic acid to these other amino acids begins with reduction of aspartic acid to its "semialdehyde," HO2CCH(NH2)CH2CHO.[2] Asparagine is derived from aspartic acid via transamidation:
- HO2CCH(NH2)CH2CO2H + GC(O)NH2 HO2CCH(NH2)CH2CONH2 + GC(O)OH
(where GC(O)NH2 and GC(O)OH are glutamine and glutamic acid, respectively)
Other biochemical roles
Aspartic acid is also a metabolite in the urea cycle and participates in gluconeogenesis. It carries reducing equivalents in the malate-aspartate shuttle, which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartic acid donates one nitrogen atom in the biosynthesis of inositol, the precursor to the purine bases.
Neurotransmitter
Aspartate (the conjugate base of aspartic acid) stimulates NMDA receptors, though not as strongly as the amino acid neurotransmitter glutamate does.[3] It serves as an excitatory neurotransmitter in the brain and is an excitotoxin.
As a neurotransmitter, aspartic acid may provide resistance to fatigue and thus lead to endurance, although the evidence to support this idea is not strong.
Synthesis
Racemic aspartic acid can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2).[4]
ReferencesISBN links support NWE through referral fees
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. Nomenclature and Symbolism for Amino Acids and Peptides. Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved 2007-05-17.
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Philip E. Chen, Matthew T. Geballe, Phillip J. Stansfeld, Alexander R. Johnston, Hongjie Yuan, Amanda L. Jacob, James P. Snyder, Stephen F. Traynelis, and David J. A. Wyllie. 2005. Structural Features of the Glutamate Binding Site in Recombinant NR1/NR2A N-Methyl-D-aspartate Receptors Determined by Site-Directed Mutagenesis and Molecular Modeling. Molecular Pharmacology. Volume 67, Pages 1470-1484.
- ↑ Dunn, M. S.; Smart, B. W. “DL-Aspartic Acid”Organic Syntheses, Collected Volume 4, p.55 (1963). http://www.orgsyn.org/orgsyn/pdfs/CV4P0055.pdf
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Kendall, E. C., and B. F. McKenzie. 1941. dl-Alanine. Organic Syntheses 1: 21.
See also
- Aspartate transaminase
- Sodium poly(aspartate), a synthetic polyamide
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.