Difference between revisions of "Apatite" - New World Encyclopedia
| (21 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{Copyedited}} |
| − | |||
| − | |||
{{Infobox mineral | {{Infobox mineral | ||
| name = Apatite | | name = Apatite | ||
| Line 12: | Line 10: | ||
| formula = Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(F,Cl,OH) | | formula = Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(F,Cl,OH) | ||
| molweight = | | molweight = | ||
| − | | color = [[Transparent]] to [[translucent]], usually green, less often colorless, yellow, blue to violet, pink, brown.<ref | + | | color = [[Transparent]] to [[translucent]], usually green, less often colorless, yellow, blue to violet, pink, brown.<ref>''GIA Gem Reference Guide''(Carlsbad, CA: [[Gemological Institute of America]], 1995), ISBN 0873110196</ref> |
| habit = Tabular, prismatic crystals, massive, compact or granular | | habit = Tabular, prismatic crystals, massive, compact or granular | ||
| − | | system = [[Hexagonal (crystal system)|Hexagonal]] Dipyramidal (6/m)<ref | + | | system = [[Hexagonal (crystal system)|Hexagonal]] Dipyramidal (6/m)<ref>B. & C. Gobin, ''Worldwide Fine Minerals by B & C Gobin''[http://webmineral.com/data/Apatite.shtml Apatite Mineral Data.] ''Webmineral.com'' Retrieved May 8, 2007.</ref> |
| twinning = | | twinning = | ||
| − | | cleavage = [0001] Indistinct, [1010] Indistinct<ref | + | | cleavage = [0001] Indistinct, [1010] Indistinct <ref>Gobin</ref> |
| − | | fracture = Conchoidal to uneven<ref | + | | fracture = Conchoidal to uneven<ref>GIA</ref> |
| − | | mohs = 5<ref | + | | mohs = 5<ref>Ibid.</ref> |
| − | | luster = Vitreous<ref | + | | luster = Vitreous<ref>Ibid.</ref> to subresinous |
| − | | polish = Vitreous<ref | + | | polish = Vitreous<ref>Ibid.</ref> |
| − | | refractive = 1.634 - 1.638 (+.012, -.006)<ref | + | | refractive = 1.634 - 1.638 (+.012, -.006)<ref>Ibid.</ref> |
| − | | opticalprop = Double refractive, uniaxial negative<ref | + | | opticalprop = Double refractive, uniaxial negative<ref>Ibid.</ref> |
| − | | birefringence = .002-.008<ref | + | | birefringence = .002-.008<ref>Ibid.</ref> |
| − | | dispersion = .013<ref | + | | dispersion = .013<ref>Ibid.</ref> |
| − | | pleochroism = ''Blue stones'' - strong, blue and yellow to colorless. Other colors are weak to very weak.<ref | + | | pleochroism = ''Blue stones'' - strong, blue and yellow to colorless. Other colors are weak to very weak.<ref>Ibid.</ref> |
| − | | fluorescence= ''Yellow stones'' - purplish pink which is stronger in long wave; ''blue stones'' - blue to light blue in both long and short wave; ''green stones'' - greenish yellow which is stronger in long wave; ''violet stones'' - greenish yellow in long wave, light purple in short wave.<ref | + | | fluorescence= ''Yellow stones'' - purplish pink which is stronger in long wave; ''blue stones'' - blue to light blue in both long and short wave; ''green stones'' - greenish yellow which is stronger in long wave; ''violet stones'' - greenish yellow in long wave, light purple in short wave.<ref>Ibid.</ref> |
| absorption = | | absorption = | ||
| streak = White | | streak = White | ||
| − | | gravity = 3.16 - 3.22<ref | + | | gravity = 3.16 - 3.22<ref>Gobin</ref> |
| density = | | density = | ||
| melt = | | melt = | ||
| Line 35: | Line 33: | ||
| diagnostic = | | diagnostic = | ||
| solubility = | | solubility = | ||
| − | | diaphaneity = Transparent to translucent<ref | + | | diaphaneity = Transparent to translucent<ref>Ibid.</ref> |
| + | |||
| other = | | other = | ||
}} | }} | ||
| − | '''Apatite''' is a group of [[phosphate minerals]], usually referring to | + | '''Apatite''' is the name given to a group of [[phosphate minerals]], usually referring to '''hydroxylapatite''' (or '''hydroxyapatite'''), '''fluoroapatite''' (or '''fluorapatite'''), and '''chloroapatite''' (or '''chlorapatite'''). They are named for the presence of hydroxide ([[Hydroxyl|OH]]<sup>-</sup>), fluoride ([[Fluorine|F]]<sup>-</sup>), and chloride ([[Chlorine|Cl]]<sup>-</sup>) [[ion]]s, respectively, in the [[crystal]] lattice. These three forms of apatite are not readily distinguishable, as each specimen usually contains all three types of ions. Impure, massive apatite is called '''phosphorite'''. |
| − | + | ||
| + | Apatite is distributed widely in [[igneous]], [[metamorphic]], and [[sedimentary]] rocks, often in the form of cryptocrystalline fragments. It is usually green, but blue, yellow, purple, and brown varieties have also been found. The crystals range from transparent to translucent, with a vitreous to greasy luster. | ||
| − | + | This mineral is also a biological material. In particular, hydroxylapatite is the main constituent of [[tooth enamel]], and a special form of apatite is found in [[bone]]. When [[toothpaste]]s and [[water]] containing [[fluoride]] are used, the fluoride ions substitute for hydroxide ions in tooth enamel, making the enamel more resistant to attack by acids. | |
| − | + | Apatite has a diverse range of uses. For example, in medicine, hydroxylapatite is used as a filler to replace amputated bone or as a coating to promote the growth of bone into [[prosthesis|prosthetic]] implants. Also, some [[dental implants]] are coated with hydroxylapatite, in the belief that it may promote integration into bone tissue. Researchers use hydroxylapatite for a [[chromatography|chromatographic]] technique to purify proteins and other chemicals. Geologists have used a [[radiometric dating]] technique (known as [[fission track dating]]) with natural deposits of apatite to get a sense of historical changes in temperature in mountain-forming belts and [[sedimentary basin]]s. In some cases, crystals of apatite have been cut and used as gems. | |
| + | {{toc}} | ||
| + | It should be noted that phosphate, arsenate, and vanadate minerals with similar crystalline structures (hexagonal or pseudohexagonal monoclinic crystals) are known as the Apatite Group. This group includes minerals such as apatite, [[mimetite]], [[pyromorphite]], and [[vanadinite]]. | ||
| − | + | == Etymology == | |
| − | [[ | + | The name ''apatite'' is derived from a Greek word that means "to deceive," because it appears similar to other minerals, particularly [[olivine]], [[beryl]], and [[peridot]]. |
| − | ''' | + | == Occurrence == |
| + | |||
| + | '''Biological:''' | ||
| + | Apatite is one of few minerals that are produced and used by biological systems. Hydroxylapatite is the main component of [[tooth enamel]]. A relatively unique form of apatite—in which most OH groups are absent and containing many carbonate and acid phosphate substitutions—is a large component of [[bone]] material. | ||
| + | |||
| + | '''Mineralogical:''' | ||
| + | In mineral form, noteworthy areas of occurrence include Bancroft, Ontario; Durango, Mexico; Germany; and Russia. | ||
| + | |||
| + | == Characteristics == | ||
| + | |||
| + | The overall chemical formula for apatite is generally given as [[Calcium|Ca]]<sub>5</sub>([[Phosphate|PO<sub>4</sub>]])<sub>3</sub>(OH, F, Cl). The formulas for the three common species may be written as: | ||
| + | * Hydroxylapatite: Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(OH) | ||
| + | * Fluoroapatite: Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>F | ||
| + | * Chlorapatite: Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>Cl | ||
| + | |||
| + | Apatite has a hardness of 5 on the [[Mohs scale]], and its [[specific gravity]] is between 3.1 and 3.2. Its crystals belong to the hexagonal crystal system, and the crystal habit is typically hexagonal prism, terminating with a hexagonal pyramid or pinacoid shape. In addition, apatite may occur in acicular (needle-like), granular, reniform, and massive forms. | ||
| + | |||
| + | == Hydroxylapatite == | ||
| + | [[Image:Mineraly.sk - hydroxylapatit.jpg|right|thumb|250px|A sample of hydroxylapatite.]] | ||
| + | |||
| + | Hydroxylapatite is the [[hydroxyl]] endmember of the apatite group. The OH<sup>-</sup> [[ion]] can be replaced by [[fluorine|fluoride]], [[chlorine|chloride]] or [[carbonate]]. As noted above, its formula may be written as Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(OH). The formula may also be written as Ca<sub>10</sub>(PO<sub>4</sub>)<sub>6</sub>(OH)<sub>2</sub>, to indicate that each crystal unit cell combines two molecules. | ||
| + | |||
| + | Purified hydroxylapatite powder is white. Naturally occurring forms can also be brown, yellow, or green. | ||
| + | |||
| + | Hydroxylapatite is the main mineral component of [[osseous tissue|bone]]. Hydroxylapatite that is deficient in carbonated calcium is the main constituent of dental enamel and dentin. | ||
== Fluoroapatite == | == Fluoroapatite == | ||
| + | [[Image:Apatite09.jpg|thumb|left|250px|Fluoroapatite crystal, Mexico.]] | ||
| + | |||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
! {{chembox header}} | Fluoroapatite | ! {{chembox header}} | Fluoroapatite | ||
| Line 93: | Line 121: | ||
| Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>OH<br/>Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>Cl | | Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>OH<br/>Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>Cl | ||
|- | |- | ||
| − | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br> materials in their [[standard state|standard state (at 25 °C, 100 kPa) | + | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br/> materials in their [[standard state|standard state (at 25 °C, 100 kPa)]]</small> |
|- | |- | ||
|} | |} | ||
| − | Fluoroapatite | + | Fluoroapatite is a hard crystalline solid that may be classified as a calcium halophosphate. The pure mineral is colorless, but naturally occurring samples can have various colors, such as green, brown, blue, or violet. It is an important constituent of [[tooth enamel]]. It is often combined as a solid solution with hydroxylapatite in biological matrices. |
| − | + | Fluoroapatite can be synthesized in a two-step process. First, [[calcium phosphate]] is generated by combining calcium and phosphate [[salt]]s at neutral [[pH]]. This material then reacts further with fluoride sources (such as [[sodium monofluorophosphate]] or [[calcium fluoride]] (CaF<sub>2</sub>)) to give the desired material. This reaction is an integral part of the global [[phosphorous cycle]].<ref>A. F. Holleman and E. Wiberg, ''Inorganic Chemistry'' (San Diego: Academic Press, 2001). ISBN 0123526515</ref> The reactions may be written as follows: | |
| − | |||
| − | Fluoroapatite can be synthesized in a two step process. | ||
:3Ca<sup>2+</sup> + 2PO<sub>4</sub><sup>3-</sup> → Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> | :3Ca<sup>2+</sup> + 2PO<sub>4</sub><sup>3-</sup> → Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> | ||
| Line 107: | Line 133: | ||
:3 Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> + CaF<sub>2</sub> → 2 Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>F | :3 Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> + CaF<sub>2</sub> → 2 Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>F | ||
| − | Fluoroapatite can also be used as a precursor for the production of [[phosphorus]]. | + | Fluoroapatite can also be used as a precursor for the production of [[phosphorus]]. The mineral can be reduced by [[carbon]] in the presence of [[quartz]], ultimately generating [[white phosphorus]] (P<sub>4</sub>), as follows: |
:Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>F + 3SiO<sub>2</sub> + 5C → 3CaSiO<sub>3</sub> + 5CO + P<sub>2</sub> | :Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>F + 3SiO<sub>2</sub> + 5C → 3CaSiO<sub>3</sub> + 5CO + P<sub>2</sub> | ||
| − | 2P<sub>2</sub> → P<sub>4</sub> after cooling | + | :2P<sub>2</sub> → P<sub>4</sub> (after cooling) |
| + | |||
| + | == Applications == | ||
| + | |||
| + | * Geologists often use a [[radiometric dating]] technique in which they follow [[Fission track dating|fission tracks]] (of [[uranium]]) in apatite to determine the thermal history of orogenic (mountain-forming) belts and sediments in [[sedimentary basin]]s. | ||
| + | |||
| + | * Fluoroapatite is more resistant to acid attack than is hydroxylapatite. For this reason, toothpastes typically contain a source of fluoride anions (such as sodium fluoride or [[sodium monofluorophosphate]]), allowing for the exchange of fluoride ions for hydroxy groups in the apatite in [[tooth|teeth]]. [[Fluoridated water]] has a similar effect. Too much fluoride, however, results in [[dental fluorosis]] or [[skeletal fluorosis]]. | ||
| + | |||
| + | * Hydroxylapatite can be used as a filler to replace amputated bone or as a coating to promote bone ingrowth into [[prosthesis|prosthetic]] implants. Although many other [[phase (matter)|phase]]s exist with similar or even identical chemical makeup, the body responds quite differently to them. [[Coral]] skeletons can be transformed into hydroxylapatite by high temperatures; their porous structure allows relatively rapid ingrowth at the expense of initial mechanical strength. The high temperature also burns away organic molecules such as [[protein]]s, preventing [[Transplant_rejection|host-versus-graft]] disease.<ref>It may be noted that bioactive glasses are the only manmade materials known to bond to both bone and soft tissue and have been clinically used as a bone grafting material for over 20 years in dental, maxillofacial, and orthopedic procedures. Unlike hydroxyapatite, which is said to be “osteoconductive” by conducting new bone growth along the materials surface, bioactive glasses are “osteostimulative” in that the material stimulates the recruitment and differentiation of osteoblasts—cells that produce new bone. As a result, bioactive glass rapidly enhances the production of new bone and is completely resorbed by the body and replaced by new bone. Bioactive glasses have also been used in oral care applications as a tooth remineralizer [[(calcium sodium phosphosilicate)]] in both professional dental and consumer oral care products.</ref> | ||
| + | |||
| + | * Some modern [[dental implants]] are coated with hydroxylapatite. It has been suggested that this may promote [[osseointegration]], but conclusive clinical proof of this has yet to come. | ||
| − | + | * Hydroxylapatite is used to purify proteins and other chemicals by the technique known as hydroxylapatite (HAP) [[chromatography]]. The mechanism involved in this technique is complicated and has been described as "mixed-mode" ion exchange. | |
| − | + | * In the United States, apatite is often used to fertilize [[tobacco]]. It partially starves the plant of nitrogen, which gives American [[cigarette]]s a different taste from those of other countries. | |
| − | [[ | + | * Apatite is infrequently used as a [[gemstone]]. Transparent stones of clean color have been faceted, and [[chatoyant]] specimens have been [[cabochon]] cut.<ref>GIA</ref> Chatoyant stones are known as ''cat's-eye apatite,''.<ref>Ibid.</ref> |
| + | transparent green stones are known as ''asparagus stone,''<ref>Ibid.</ref> and blue stones may be called ''[[moroxite]].''<ref>Edwin W. Streeter, [http://www.farlang.com/gemstones/streeter-precious-stones/page_306/view?searchterm=moroxite Moroxite] ''Precious Stones and Gems,'' 6th ed. (London: George Bell and Sons, 1898), 306. Retrieved May 8, 2007.</ref> If crystals of rutile have grown in the apatite crystal, the cut stone displays a cat's eye effect when viewed in the right lighting. Major sources<ref>GIA</ref> for gem-quality apatite are: [[Brazil]], [[Burma]], and [[Mexico]]. Additional sources include [[Canada]], [[Czechoslovakia]], [[Germany]], [[India]], [[Madagascar]], [[Mozambique]], [[Norway]], [[South Africa]], [[Spain]], [[Sri Lanka]], and the [[United States]]. | ||
== See also == | == See also == | ||
| Line 130: | Line 167: | ||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Farndon, John. 2006. ''The Practical Encyclopedia of Rocks & Minerals: How to Find, Identify, Collect and Maintain the World's best Specimens, with over 1000 Photographs and Artworks.'' London: Lorenz Books. ISBN 0754815412 |
| + | * Gobin, B. & C.Worldwide Fine Minerals by B & C GOBIN[http://webmineral.com/data/Apatite.shtml Apatite Mineral Data.] ''Webmineral.com''. Retrieved May 8, 2007. | ||
| + | * Holleman, A. F. and E. Wiberg, Inorganic Chemistry (San Diego: Academic Press, 2001). ISBN 0123526515 | ||
| + | * Klein, Cornelis, and Barbara Dutrow. 2007. ''Manual of Mineral Science,'' 23rd ed. New York: John Wiley. ISBN 9780471721574 | ||
| + | * Pellant, Chris. 2002. ''Rocks and Minerals.'' Smithsonian Handbooks. New York: Dorling Kindersley. ISBN 0789491060 | ||
| + | * Shaffer, Paul R., Herbert S. Zim, and Raymond Perlman. 2001. ''Rocks, Gems and Minerals,'' Rev. ed. New York: St. Martin's Press. ISBN 1582381321 | ||
| + | * Streeter, Edwin W. ''Moroxite Precious Stones and Gems,'' 6th ed. (London: George Bell and Sons, 1898), 306. Retrieved May 8, 2007. | ||
| + | * Mineral Gallery. 2006. The Mineral Apatite. ''Amethyst Galleries.'' | ||
== External links == | == External links == | ||
| + | All links retrieved August 11, 2023. | ||
| − | * [http://www.mindat.org/min-29229.html Apatite.] ''Mindat.org'' | + | * [http://www.mindat.org/min-29229.html Apatite.] ''Mindat.org''. |
| − | + | * [http://www.mindat.org/min-274.html Apatite Supergroup.] ''Mindat.org''. | |
| − | * [http://www.mindat.org/min-274.html Apatite | + | *[http://www.mindat.org/min-1992.html Hydroxylapatite.] ''Mindat.org''. |
| − | + | * Worldwide Fine Minerals by B & C GOBIN [http://webmineral.com/data/Hydroxylapatite.shtml Hydroxylapatite Mineral Data.] ''Webmineral.com''. | |
| − | * [http://webmineral.com/data/ | + | * Worldwide Fine Minerals by B & C GOBIN [http://webmineral.com/data/Fluorapatite.shtml Fluoroapatite Mineral Data.] ''Webmineral.com''. |
| + | *[http://www.azom.com/details.asp?ArticleID=107 Hydroxyapatite.] ''Azom.com''. | ||
| − | |||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
| Line 157: | Line 193: | ||
[[Category:Minerals]] | [[Category:Minerals]] | ||
| − | {{credits|Apatite|123652172|Fluoroapatite|124859012}} | + | {{credits|Apatite|123652172|Fluoroapatite|124859012|Hydroxylapatite|126179894}} |
Latest revision as of 05:59, 11 August 2023
| Apatite | |
|---|---|
 |
|
| General | |
| Category | Phosphate mineral group |
| Chemical formula | Ca5(PO4)3(F,Cl,OH) |
| Identification | |
| Color | Transparent to translucent, usually green, less often colorless, yellow, blue to violet, pink, brown.[1] |
| Crystal habit | Tabular, prismatic crystals, massive, compact or granular |
| Crystal system | Hexagonal Dipyramidal (6/m)[2] |
| Cleavage | [0001] Indistinct, [1010] Indistinct [3] |
| Fracture | Conchoidal to uneven[4] |
| Mohs Scale hardness | 5[5] |
| Luster | Vitreous[6] to subresinous |
| Refractive index | 1.634 - 1.638 (+.012, -.006)[7] |
| Optical Properties | Double refractive, uniaxial negative[8] |
| Birefringence | .002-.008[9] |
| Pleochroism | Blue stones - strong, blue and yellow to colorless. Other colors are weak to very weak.[10] |
| Streak | White |
| Specific gravity | 3.16 - 3.22[11] |
| Diaphaneity | Transparent to translucent[12] |
Apatite is the name given to a group of phosphate minerals, usually referring to hydroxylapatite (or hydroxyapatite), fluoroapatite (or fluorapatite), and chloroapatite (or chlorapatite). They are named for the presence of hydroxide (OH-), fluoride (F-), and chloride (Cl-) ions, respectively, in the crystal lattice. These three forms of apatite are not readily distinguishable, as each specimen usually contains all three types of ions. Impure, massive apatite is called phosphorite.
Apatite is distributed widely in igneous, metamorphic, and sedimentary rocks, often in the form of cryptocrystalline fragments. It is usually green, but blue, yellow, purple, and brown varieties have also been found. The crystals range from transparent to translucent, with a vitreous to greasy luster.
This mineral is also a biological material. In particular, hydroxylapatite is the main constituent of tooth enamel, and a special form of apatite is found in bone. When toothpastes and water containing fluoride are used, the fluoride ions substitute for hydroxide ions in tooth enamel, making the enamel more resistant to attack by acids.
Apatite has a diverse range of uses. For example, in medicine, hydroxylapatite is used as a filler to replace amputated bone or as a coating to promote the growth of bone into prosthetic implants. Also, some dental implants are coated with hydroxylapatite, in the belief that it may promote integration into bone tissue. Researchers use hydroxylapatite for a chromatographic technique to purify proteins and other chemicals. Geologists have used a radiometric dating technique (known as fission track dating) with natural deposits of apatite to get a sense of historical changes in temperature in mountain-forming belts and sedimentary basins. In some cases, crystals of apatite have been cut and used as gems.
It should be noted that phosphate, arsenate, and vanadate minerals with similar crystalline structures (hexagonal or pseudohexagonal monoclinic crystals) are known as the Apatite Group. This group includes minerals such as apatite, mimetite, pyromorphite, and vanadinite.
Etymology
The name apatite is derived from a Greek word that means "to deceive," because it appears similar to other minerals, particularly olivine, beryl, and peridot.
Occurrence
Biological: Apatite is one of few minerals that are produced and used by biological systems. Hydroxylapatite is the main component of tooth enamel. A relatively unique form of apatite—in which most OH groups are absent and containing many carbonate and acid phosphate substitutions—is a large component of bone material.
Mineralogical: In mineral form, noteworthy areas of occurrence include Bancroft, Ontario; Durango, Mexico; Germany; and Russia.
Characteristics
The overall chemical formula for apatite is generally given as Ca5(PO4)3(OH, F, Cl). The formulas for the three common species may be written as:
- Hydroxylapatite: Ca5(PO4)3(OH)
- Fluoroapatite: Ca5(PO4)3F
- Chlorapatite: Ca5(PO4)3Cl
Apatite has a hardness of 5 on the Mohs scale, and its specific gravity is between 3.1 and 3.2. Its crystals belong to the hexagonal crystal system, and the crystal habit is typically hexagonal prism, terminating with a hexagonal pyramid or pinacoid shape. In addition, apatite may occur in acicular (needle-like), granular, reniform, and massive forms.
Hydroxylapatite
Hydroxylapatite is the hydroxyl endmember of the apatite group. The OH- ion can be replaced by fluoride, chloride or carbonate. As noted above, its formula may be written as Ca5(PO4)3(OH). The formula may also be written as Ca10(PO4)6(OH)2, to indicate that each crystal unit cell combines two molecules.
Purified hydroxylapatite powder is white. Naturally occurring forms can also be brown, yellow, or green.
Hydroxylapatite is the main mineral component of bone. Hydroxylapatite that is deficient in carbonated calcium is the main constituent of dental enamel and dentin.
Fluoroapatite
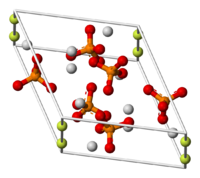
| Fluoroapatite | |
|---|---|
| |
| General | |
| Systematic name | Fluoroapatite |
| Other names | Fluorapatite |
| Molecular formula | Ca5(PO4)3F |
| Molar mass | 504.3 g/mol |
| Appearance | hard solid, various colors |
| CAS number | 68877-08-7 |
| Properties | |
| Solubility in water | almost insoluble |
| Structure | |
| Crystal structure | hexagonal |
| Related compounds | |
| Related compounds | Ca5(PO4)3OH Ca5(PO4)3Cl |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Fluoroapatite is a hard crystalline solid that may be classified as a calcium halophosphate. The pure mineral is colorless, but naturally occurring samples can have various colors, such as green, brown, blue, or violet. It is an important constituent of tooth enamel. It is often combined as a solid solution with hydroxylapatite in biological matrices.
Fluoroapatite can be synthesized in a two-step process. First, calcium phosphate is generated by combining calcium and phosphate salts at neutral pH. This material then reacts further with fluoride sources (such as sodium monofluorophosphate or calcium fluoride (CaF2)) to give the desired material. This reaction is an integral part of the global phosphorous cycle.[13] The reactions may be written as follows:
- 3Ca2+ + 2PO43- → Ca3(PO4)2
- 3 Ca3(PO4)2 + CaF2 → 2 Ca5(PO4)3F
Fluoroapatite can also be used as a precursor for the production of phosphorus. The mineral can be reduced by carbon in the presence of quartz, ultimately generating white phosphorus (P4), as follows:
- Ca5(PO4)3F + 3SiO2 + 5C → 3CaSiO3 + 5CO + P2
- 2P2 → P4 (after cooling)
Applications
- Geologists often use a radiometric dating technique in which they follow fission tracks (of uranium) in apatite to determine the thermal history of orogenic (mountain-forming) belts and sediments in sedimentary basins.
- Fluoroapatite is more resistant to acid attack than is hydroxylapatite. For this reason, toothpastes typically contain a source of fluoride anions (such as sodium fluoride or sodium monofluorophosphate), allowing for the exchange of fluoride ions for hydroxy groups in the apatite in teeth. Fluoridated water has a similar effect. Too much fluoride, however, results in dental fluorosis or skeletal fluorosis.
- Hydroxylapatite can be used as a filler to replace amputated bone or as a coating to promote bone ingrowth into prosthetic implants. Although many other phases exist with similar or even identical chemical makeup, the body responds quite differently to them. Coral skeletons can be transformed into hydroxylapatite by high temperatures; their porous structure allows relatively rapid ingrowth at the expense of initial mechanical strength. The high temperature also burns away organic molecules such as proteins, preventing host-versus-graft disease.[14]
- Some modern dental implants are coated with hydroxylapatite. It has been suggested that this may promote osseointegration, but conclusive clinical proof of this has yet to come.
- Hydroxylapatite is used to purify proteins and other chemicals by the technique known as hydroxylapatite (HAP) chromatography. The mechanism involved in this technique is complicated and has been described as "mixed-mode" ion exchange.
- In the United States, apatite is often used to fertilize tobacco. It partially starves the plant of nitrogen, which gives American cigarettes a different taste from those of other countries.
- Apatite is infrequently used as a gemstone. Transparent stones of clean color have been faceted, and chatoyant specimens have been cabochon cut.[15] Chatoyant stones are known as cat's-eye apatite,.[16]
transparent green stones are known as asparagus stone,[17] and blue stones may be called moroxite.[18] If crystals of rutile have grown in the apatite crystal, the cut stone displays a cat's eye effect when viewed in the right lighting. Major sources[19] for gem-quality apatite are: Brazil, Burma, and Mexico. Additional sources include Canada, Czechoslovakia, Germany, India, Madagascar, Mozambique, Norway, South Africa, Spain, Sri Lanka, and the United States.
See also
Notes
- ↑ GIA Gem Reference Guide(Carlsbad, CA: Gemological Institute of America, 1995), ISBN 0873110196
- ↑ B. & C. Gobin, Worldwide Fine Minerals by B & C GobinApatite Mineral Data. Webmineral.com Retrieved May 8, 2007.
- ↑ Gobin
- ↑ GIA
- ↑ Ibid.
- ↑ Ibid.
- ↑ Ibid.
- ↑ Ibid.
- ↑ Ibid.
- ↑ Ibid.
- ↑ Gobin
- ↑ Ibid.
- ↑ A. F. Holleman and E. Wiberg, Inorganic Chemistry (San Diego: Academic Press, 2001). ISBN 0123526515
- ↑ It may be noted that bioactive glasses are the only manmade materials known to bond to both bone and soft tissue and have been clinically used as a bone grafting material for over 20 years in dental, maxillofacial, and orthopedic procedures. Unlike hydroxyapatite, which is said to be “osteoconductive” by conducting new bone growth along the materials surface, bioactive glasses are “osteostimulative” in that the material stimulates the recruitment and differentiation of osteoblasts—cells that produce new bone. As a result, bioactive glass rapidly enhances the production of new bone and is completely resorbed by the body and replaced by new bone. Bioactive glasses have also been used in oral care applications as a tooth remineralizer (calcium sodium phosphosilicate) in both professional dental and consumer oral care products.
- ↑ GIA
- ↑ Ibid.
- ↑ Ibid.
- ↑ Edwin W. Streeter, Moroxite Precious Stones and Gems, 6th ed. (London: George Bell and Sons, 1898), 306. Retrieved May 8, 2007.
- ↑ GIA
ReferencesISBN links support NWE through referral fees
- Farndon, John. 2006. The Practical Encyclopedia of Rocks & Minerals: How to Find, Identify, Collect and Maintain the World's best Specimens, with over 1000 Photographs and Artworks. London: Lorenz Books. ISBN 0754815412
- Gobin, B. & C.Worldwide Fine Minerals by B & C GOBINApatite Mineral Data. Webmineral.com. Retrieved May 8, 2007.
- Holleman, A. F. and E. Wiberg, Inorganic Chemistry (San Diego: Academic Press, 2001). ISBN 0123526515
- Klein, Cornelis, and Barbara Dutrow. 2007. Manual of Mineral Science, 23rd ed. New York: John Wiley. ISBN 9780471721574
- Pellant, Chris. 2002. Rocks and Minerals. Smithsonian Handbooks. New York: Dorling Kindersley. ISBN 0789491060
- Shaffer, Paul R., Herbert S. Zim, and Raymond Perlman. 2001. Rocks, Gems and Minerals, Rev. ed. New York: St. Martin's Press. ISBN 1582381321
- Streeter, Edwin W. Moroxite Precious Stones and Gems, 6th ed. (London: George Bell and Sons, 1898), 306. Retrieved May 8, 2007.
- Mineral Gallery. 2006. The Mineral Apatite. Amethyst Galleries.
External links
All links retrieved August 11, 2023.
- Apatite. Mindat.org.
- Apatite Supergroup. Mindat.org.
- Hydroxylapatite. Mindat.org.
- Worldwide Fine Minerals by B & C GOBIN Hydroxylapatite Mineral Data. Webmineral.com.
- Worldwide Fine Minerals by B & C GOBIN Fluoroapatite Mineral Data. Webmineral.com.
- Hydroxyapatite. Azom.com.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.

