Disaccharide
A disaccharide is a sugar (a carbohydrate) composed of two monosaccharides.[1]
'Disaccharide' is one of the four chemical groupings of carbohydrates (monosaccharide, disaccharide, oligosaccharide, and polysaccharide).
The general chemical formula for carbohydrates, C(H2O), gives the relative proportions of carbon, hydrogen, and oxygen in a monosaccharide (the proportion of these atoms are 1:2:1). This formula is characteristic of sugars and gave rise to the term carbohydrate because compounds of this sort were originally thought to be "hydrates of carbon." This term persists even though a carbohydrate definitely is not a hydrated carbon atom. In disaccharides, oligosaccharides, and polysaccharides, the molar proportions deviate slightly from the general formula because two hydrogens and one oxygen are lost during each of the condensation reactions that forms them. These carbohydrates have the more general formula Cn(H2O)m.
Formation
It is formed when two sugars are joined together and a molecule of water is removed. For example, milk sugar (lactose) is made from glucose and galactose whereas cane sugar (sucrose) is made from glucose and fructose.
The two monosaccharides are bonded via a dehydration reaction (also called a condensation reaction) that leads to the loss of a molecule of water.
Structure
Carbohydrates are a class of biological molecules that contain primarily carbon (C) atoms flanked by hydrogen (H) atoms and hydroxyl (OH) groups (H-C-OH).
The repeating units of polysaccharides are simple sugars called monosaccharides. There are two categories of sugars: aldosugars, with a terminal carbonyl group (a carbon atom double-bonded to an oxygen atom), and ketosugars, with an internal carbonyl group typically on the second carbon atom.
Within these two groups, sugars are named according to the number of carbon atoms they contain. Most sugars have between three and seven carbon atoms are termed triose (three carbons), tetrose (four carbons), pentose (five carbons), hexose (six carbons), or heptose (seven carbons).
Glucose is an aldohexose, fructose is a ketohexose, and ribose is an aldopentose. Each carbon atom that supports a hydroxyl group (except for the first and last) is optically active, allowing a number of different carbohydrates with the same basic structure. For instance, galactose is an aldohexose but has different properties from glucose because the atoms are arranged differently. The single most common monosaccharide is the aldohexose D-glucose, represented by the formula C6H12O6. The carbons of glucose are numbered beginning with the more oxidized end of the molecule, the carbonyl group.
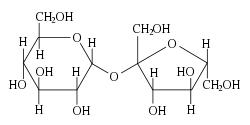
In addition to the free monosaccharide, glucose also occurs in disaccharides, which consist of two monosaccharide units linked covalently. Each disaccharide is formed by a condensation reaction in which there is a loss of hydrogen (H) from one molecule and a hydroxyl group (OH) from the other. Glycosidic bonds form between carbon 1 of the first glucose molecule and carbon 4 of the second glucose molecule. The resulting glycosidic bond is the characteristic linkage between sugars. Three common disaccharides are shown in the figure. Maltose (malt sugar) is made up of two glucose units linked together. Lactose (milk sugar) consists of a glucose linked to a galactose. Sucrose (common table sugar) has a glucose linked to a fructose.
Although the disaccharide maltose contains two glucose molecules, it is not the only disaccharide that can be made from two glucoses. When glucose molecules form a glycosidic bond, the linkage will be one of two types, α or β, depending on whether the molecule that bonds its carbon 1 is an α-glucose or β-glucose. An α-linkage with carbon 4 of a second glucose molecule results in maltose, whereas a β-linkage results in cellobiose. Although maltose and cellobiose are disaccharide isomers, both having the formula C12H22O11, they are different compounds with different properties. For example, maltose can be hydrolyzed to its monosaccharides in the human body where as cellobiose cannot. Some organisms have the capacity to break down cellobiose.
Properties
The glycosidic bond can be formed between any hydroxyl group on the component monosaccharide. So, even if both component sugars are the same (e.g., glucose), different bond combinations (regiochemistry) and stereochemistry (alpha- or beta-) result in disaccharides that are diastereoisomers with different chemical and physical properties.
Depending on the monosaccharide constituents, disaccharides are sometimes crystalline, sometimes water-soluble, and sometimes sweet-tasting.
Common disaccharides
| Disaccharide | Unit 1 | Unit 2 | Bond | Disaccharidase |
| Sucrose (table sugar, cane sugar, saccharose, or beet sugar) | glucose | fructose | α(1→2) | sucrase |
| Lactose (milk sugar) | galactose | glucose | β(1→4) | lactase |
| Maltose | glucose | glucose | α(1→4) | maltase |
| Trehalose | glucose | glucose | α(1→1)α | trehalase |
| Cellobiose | glucose | glucose | β(1→4) | cellobiase |
Maltose and cellobiose are hydrolysis products of the polysaccharides, starch and cellulose, respectively.
ReferencesISBN links support NWE through referral fees
- ↑ International Union of Pure and Applied Chemistry. "disaccharides". Compendium of Chemical Terminology Internet edition.
External links
- MeSH Disaccharides
Template:Chemistry-stub
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.