Difference between revisions of "Carotenoid" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 1: | Line 1: | ||
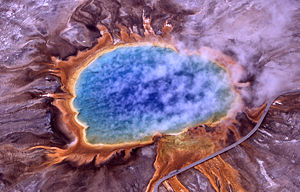
[[Image:Grand prismatic spring.jpg|thumb|300px|The orange ring surrounding [[Grand Prismatic Spring]] is due to carotenoid molecules, produced by huge mats of [[algae]] and [[bacteria]].]] | [[Image:Grand prismatic spring.jpg|thumb|300px|The orange ring surrounding [[Grand Prismatic Spring]] is due to carotenoid molecules, produced by huge mats of [[algae]] and [[bacteria]].]] | ||
| − | ''' | + | '''Carotenoid''' is any of a large class of over 600 [[organic compound|organic]] [[pigment]]s, including the carotenes and xanthophylls, that are widely distributed in nature and typically impart yellow, orange, red, or purple colors. Generally they are fat-soluble, dissolving in fats and oils but not water, except when complexed with proteins. In plants, they naturally occur in [[chromoplast]]s , imparting color to fruits and vegetables, such as carrots, pumpkins, sweet potatoes, and tomatoes. They also are found in some other [[photosynthesis|photosynthetic]] [[organism]]s like [[algae]], some types of [[fungus]], and some [[bacterium|bacteria]]. In animals such as crustaceans, nudibranches, and echinoderms, carotenoprotein complexes give red, purple, green, blue, and other colors. |
| − | + | ||
| + | Carotenoids serve two key roles in plants and algae: they absorb light energy for use in photosynthesis, and they protect chlorophyll from photodamage (Armstrong and Hearst 1996). In humans, carotenoids such as [[beta-carotene]] are a precursor to [[vitamin A]], a pigment essential for good vision, and carotenoids can also act as [[antioxidant]]s (Sims and Odle 2005). | ||
| + | |||
| + | ==Overview== | ||
| + | |||
| + | they are split into two classes, [[xanthophyll]]s and [[carotene]]s. They absorb blue light. | ||
People consuming diets rich in carotenoids from natural foods, such as fruits and vegetables, are healthier and have lower mortality from a number of chronic illnesses.{{Fact|date=June 2008}} However, a recent meta-analysis of 68 reliable antioxidant supplementation experiments involving a total of 232,606 individuals concluded that consuming additional beta-carotene from supplements is unlikely to be beneficial and may actually be harmful,<ref>{{cite journal |author=Bjelakovic G, et al |title=Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis |journal=JAMA |volume=297 |issue=8 |pages=842–57 |year=2007 |pmid=17327526 |doi=10.1001/jama.297.8.842}}</ref> although this conclusion may be due to the inclusion of studies involving smokers.<ref> | People consuming diets rich in carotenoids from natural foods, such as fruits and vegetables, are healthier and have lower mortality from a number of chronic illnesses.{{Fact|date=June 2008}} However, a recent meta-analysis of 68 reliable antioxidant supplementation experiments involving a total of 232,606 individuals concluded that consuming additional beta-carotene from supplements is unlikely to be beneficial and may actually be harmful,<ref>{{cite journal |author=Bjelakovic G, et al |title=Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis |journal=JAMA |volume=297 |issue=8 |pages=842–57 |year=2007 |pmid=17327526 |doi=10.1001/jama.297.8.842}}</ref> although this conclusion may be due to the inclusion of studies involving smokers.<ref> | ||
| Line 162: | Line 167: | ||
**[[Bacterioruberin]] 2,2'-Bis(3-hydroxy-3-methylbutyl)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-y,y-carotene-1,1'-dio | **[[Bacterioruberin]] 2,2'-Bis(3-hydroxy-3-methylbutyl)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-y,y-carotene-1,1'-dio | ||
| − | ==References==< | + | ==References== |
| − | + | ||
| + | .<ref>{{cite journal |author=Armstrong GA, Hearst JE |title=Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis |journal=FASEB J. |volume=10 |issue=2 |pages=228–37 |year=1996 |pmid=8641556 |doi= |url=http://www.fasebj.org/cgi/pmidlookup?view=long&pmid=8641556}}}</ref> | ||
Revision as of 01:42, 10 September 2008
Carotenoid is any of a large class of over 600 organic pigments, including the carotenes and xanthophylls, that are widely distributed in nature and typically impart yellow, orange, red, or purple colors. Generally they are fat-soluble, dissolving in fats and oils but not water, except when complexed with proteins. In plants, they naturally occur in chromoplasts , imparting color to fruits and vegetables, such as carrots, pumpkins, sweet potatoes, and tomatoes. They also are found in some other photosynthetic organisms like algae, some types of fungus, and some bacteria. In animals such as crustaceans, nudibranches, and echinoderms, carotenoprotein complexes give red, purple, green, blue, and other colors.
Carotenoids serve two key roles in plants and algae: they absorb light energy for use in photosynthesis, and they protect chlorophyll from photodamage (Armstrong and Hearst 1996). In humans, carotenoids such as beta-carotene are a precursor to vitamin A, a pigment essential for good vision, and carotenoids can also act as antioxidants (Sims and Odle 2005).
Overview
they are split into two classes, xanthophylls and carotenes. They absorb blue light.
People consuming diets rich in carotenoids from natural foods, such as fruits and vegetables, are healthier and have lower mortality from a number of chronic illnesses.[citation needed] However, a recent meta-analysis of 68 reliable antioxidant supplementation experiments involving a total of 232,606 individuals concluded that consuming additional beta-carotene from supplements is unlikely to be beneficial and may actually be harmful,[1] although this conclusion may be due to the inclusion of studies involving smokers.[2] Since most carotenoid-rich fruits and vegetables are low in lipids and since dietary lipids have been hypothesized to be an important factor for carotenoid bioavailability, a 2005 study investigated whether addition of avocado fruit or oil, as lipid sources, would enhance carotenoid absorption in humans. The study found that the addition of both avocado fruit and oil significantly enhanced the subjects' absorption of all carotenoids tested (alpha-carotene, beta-carotene, lycopene, and lutein).[3]
Properties
Carotenoids belong to the category of tetraterpenoids (i.e. they contain 40 carbon atoms). Structurally they are in the form of a polyene chain which is sometimes terminated by rings.
- Carotenoids with molecules containing oxygen, such as lutein and zeaxanthin, are known as xanthophylls.
- The unoxygenated (oxygen free) carotenoids such as alpha-carotene, beta-carotene and lycopene are known as carotenes. Carotenes typically contain only carbon and hydrogen.
Probably the most well-known carotenoid is the one that gives this second group its name, carotene, found in carrots (also apricots) and responsible for their bright orange colour. Crude palm oil, however, is the richest source of carotenoids in nature[4].
Their colour, ranging from pale yellow through bright orange to deep red, is directly linked to their structure. Xanthophylls are often yellow, hence their class name. The double carbon-carbon bonds interact with each other in a process called conjugation, which allows electrons in the molecule to move freely across these areas of the molecule. As the number of double bonds increases, electrons associated with conjugated systems have more room to move, and require less energy to change states. This causes the range of energies of light absorbed by the molecule to decrease. As more frequencies of light are absorbed from the short end of the visible spectrum, the compounds acquire an increasingly red appearance.[citation needed]
Physiological effects
In photosynthetic organisms, carotenoids play a vital role in the photosynthetic reaction centre. They either participate in the energy-transfer process, or protect the reaction center from auto-oxidation. In non-photosynthesizing organisms, carotenoids have been linked to oxidation-preventing mechanisms.
Carotenoids have many physiological functions. Given their structure (above), carotenoids are efficient free-radical scavengers, and they enhance the vertebrate immune system. Consequently, epidemiological studies have shown that people with high beta-carotene intake and high plasma levels of beta-carotene have a significantly reduced risk of lung cancer. However, studies of supplementation with large doses of beta-carotene in smokers have shown an increase in cancer risk (possibly because excessive beta-carotene results in breakdown products that reduce plasma vitamin A and worsen the lung cell proliferation induced by smoke[5]). Similar results have been found in other animals. Not all carotenoids are helpful, e.g. etretinate is a teratogen.
Animals are incapable of synthesizing carotenoids, and must obtain them through their diet, yet they are common and often in ornamental features. For example, the pink colour of flamingos and salmon, and the red colouring of lobsters are due to carotenoids. It has been proposed that carotenoids are used in ornamental traits because, given their physiological and chemical properties, they can be used as honest indicators of individual health, and hence they can be used by animals when selecting potential mates.
The most common carotenoids include lycopene and the vitamin A precursor β-carotene. In plants, the xanthophyll lutein is the most abundant carotenoid and its role in preventing age-related eye disease is currently under investigation. Lutein and the other carotenoid pigments found in leaves are not obvious because of the presence of other pigments such as chlorophyll.
Aroma chemicals
Products of carotenoid degradation such as ionones, damascones, and damascenones are also important fragrance chemicals that are used extensively in the perfumes and fragrance industry. Both beta-damascenone and beta-ionone although low in concentration in rose distillates are the key odour-contributing compounds in flowers. In fact, the sweet floral smells present in black tea, aged tobacco, grape, and many fruits are due to the aromatic compounds resulting from carotenoid breakdown.
Disease
Despite being important in nutrition, some carotenoids are produced by bacteria to protect themselves from immune attack, such as MRSA. The golden pigment of S. aureus allows it to survive competitive attack by Lactobaccillus as well as the human immune system.[6]
List of Naturally occurring carotenoids
- Hydrocarbons
- Lycopersene 7,8,11,12,15,7',8',11',12',15'-Decahydro-y,y-carotene
- Phytofluene
- Hexahydrolycopene 15-cis-7,8,11,12,7',8'-Hexahydro-y,y-carotene
- Torulene 3',4'-Didehydro-b,y-carotene
- a-Zeacarotene 7',8'-Dihydro-e,y-carotene
- Alcohols
- Alloxanthin
- Cynthiaxanthin
- Pectenoxanthin
- Cryptomonaxanthin (3R,3'R)-7,8,7',8'-Tetradehydro-b,b-carotene-3,3'-diol
- Crustaxanthin b,b-Carotene-3,4,3',4'-tetrol
- Gazaniaxanthin (3R)-5'-cis-b,y-Caroten-3-ol
- OH-Chlorobactene 1',2'-Dihydro-f,y-caroten-1'-ol
- Loroxanthin b,e-Carotene-3,19,3'-triol
- Lycoxanthin y,y-Caroten-16-ol
- Rhodopin 1,2-Dihydro-y,y-caroten-l-ol
- Rhodopinol aka Warmingol 13-cis-1,2-Dihydro-y,y-carotene-1,20-diol
- Saproxanthin 3',4'-Didehydro-1',2'-dihydro-b,y-carotene-3,1'-diol
- Glycosides
- Oscillaxanthin 2,2'-Bis(b-L-rhamnopyranosyloxy)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-y,y-carotene-1,1'-diol
- Phleixanthophyll 1'-(b-D-Glucopyranosyloxy)-3',4'-didehydro-1',2'-dihydro-b,y-caroten-2'-ol
- Ethers
- Rhodovibrin 1'-Methoxy-3',4'-didehydro-1,2,1',2'-tetrahydro-y,y-caroten-1-ol
- Spheroidene 1-Methoxy-3,4-didehydro-1,2,7',8'-tetrahydro-y,y-carotene
- Epoxides
- Diadinoxanthin 5,6-Epoxy-7',8'-didehydro-5,6-dihydro—carotene-3,3-diol
- Luteoxanthin 5,6: 5',8'-Diepoxy-5,6,5',8'-tetrahydro-b,b-carotene-3,3'-diol
- Mutatoxanthin
- Citroxanthin
- Zeaxanthin furanoxide 5,8-Epoxy-5,8-dihydro-b,b-carotene-3,3'-diol
- Neochrome 5',8'-Epoxy-6,7-didehydro-5,6,5',8'-tetrahydro-b,b-carotene-3,5,3'-triol
- Foliachrome
- Trollichrome
- Vaucheriaxanthin 5',6'-Epoxy-6,7-didehydro-5,6,5',6'-tetrahydro-b,b-carotene-3,5,19,3'-tetrol
- Aldehydes
- Rhodopinal
- Wamingone 13-cis-1-Hydroxy-1,2-dihydro-y,y-caroten-20-al
- Torularhodinaldehyde 3',4'-Didehydro-b,y-caroten-16'-al
- Acids and Acid Esters
- Torularhodin 3',4'-Didehydro-b,y-caroten-16'-oic acid
- Torularhodin methyl ester Methyl 3',4'-didehydro-b,y-caroten-16'-oate
- Ketones
- Canthaxanthin aka Aphanicin, Chlorellaxanthin b,b-Carotene-4,4'-dione
- Capsanthin (3R,3'S,5'R)-3,3'-Dihydroxy-b,k-caroten-6'-one
- Capsorubin (3S,5R,3'S,5'R)-3,3'-Dihydroxy-k,k-carotene-6,6'-dione
- Cryptocapsin (3'R,5'R)-3'-Hydroxy-b,k-caroten-6'-one
2,2'-Diketospirilloxanthin 1,1'-Dimethoxy-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-y,y-carotene-2,2'-dione
- Flexixanthin 3,1'-Dihydroxy-3',4'-didehydro-1',2'-dihydro-b,y-caroten-4-one
- 3-OH-Canthaxanthin aka Adonirubin aka Phoenicoxanthin 3-Hydroxy-b,b-carotene-4,4'-dione
- Hydroxyspheriodenone 1'-Hydroxy-1-methoxy-3,4-didehydro-1,2,1',2',7',8'-hexahydro-y,y-caroten-2-one
- Okenone 1'-Methoxy-1',2'-dihydro-c,y-caroten-4'-one
- Pectenolone 3,3'-Dihydroxy-7',8'-didehydro-b,b-caroten-4-one
- Phoeniconone aka Dehydroadonirubin 3-Hydroxy-2,3-didehydro-b,b-carotene-4,4'-dione
- Phoenicopterone b,e-caroten-4-one
- Rubixanthone 3-Hydroxy-b,y-caroten-4'-one
- Siphonaxanthin 3,19,3'-Trihydroxy-7,8-dihydro-b,e-caroten-8-one
- Esters of Alcohols
- Astacein 3,3'-Bispalmitoyloxy-2,3,2',3'-tetradehydro-b,b-carotene-4,4'-dione or
- 3,3'-dihydroxy-2,3,2',3'-tetradehydro-b,b-carotene-4,4'-dione dipalmitate
- Fucoxanthin 3'-Acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-didehydro-5,6,7,8,5',6'-hexahydro-b,b-caroten-8-one
- Isofucoxanthin 3'-Acetoxy-3,5,5'-trihydroxy-6',7'-didehydro-5,8,5',6'-tetrahydro-b,b-caroten-8-one
- Physalien
- Zeaxanthin dipalmitate (3R,3'R)-3,3'-Bispalmitoyloxy-b,b-carotene or
(3R,3'R)-b,b-carotene-3,3'-diol dipalmitate
- Siphonein 3,3'-Dihydroxy-19-lauroyloxy-7,8-dihydro-b,e-caroten-8-one or
3,19,3'-trihydroxy-7,8-dihydro-b,e-caroten-8-one 19-laurate
- Apo Carotenoids
- b-Apo-2'-carotenal 3',4'-Didehydro-2'-apo-b-caroten-2'-al
- Apo-2-lycopenal
- Apo-6'-lycopenal 6'-Apo-y-caroten-6'-al
- Azafrinaldehyde 5,6-Dihydroxy-5,6-dihydro-10'-apo-b-caroten-10'-al
- Bixin 6'-Methyl hydrogen 9'-cis-6,6'-diapocarotene-6,6'-dioate
- Citranaxanthin 5',6'-Dihydro-5'-apo-b-caroten-6'-one or
5',6'-dihydro-5'-apo-18'-nor-b-caroten-6'-one or 6'-methyl-6'-apo-b-caroten-6'-one
- Crocetin 8,8'-Diapo-8,8'-carotenedioic acid
- Crocetinsemialdehyde 8'-Oxo-8,8'-diapo-8-carotenoic acid
- Crocin Digentiobiosyl 8,8'-diapo-8,8'-carotenedioate
- Hopkinsiaxanthin 3-Hydroxy-7,8-didehydro-7',8'-dihydro-7'-apo-b-carotene-4,8'-dione or
3-hydroxy-8'-methyl-7,8-didehydro-8'-apo-b-carotene-4,8'-dione
- Methyl apo-6'-lycopenoate Methyl 6'-apo-y-caroten-6'-oate
- Paracentrone 3,5-Dihydroxy-6,7-didehydro-5,6,7',8'-tetrahydro-7'-apo-b-caroten-8'-one or 3,5-dihydroxy-8'-methyl-6,7-didehydro-5,6-dihydro-8'-apo-b-caroten-8'-one
- Sintaxanthin 7',8'-Dihydro-7'-apo-b-caroten-8'-one or 8'-methyl-8'-apo-b-caroten-8'-one
- Nor and Seco Carotenoids
- Actinioerythrin 3,3'-Bisacyloxy-2,2'-dinor-b,b-carotene-4,4'-dione
- b-Carotenone 5,6:5',6'-Diseco-b,b-carotene-5,6,5',6'-tetrone
- Peridinin 3'-Acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-didehydro-5,6,5',6'-tetrahydro-12',13',20'-trinor-b,b-caroten-19,11-olide
- Pyrrhoxanthininol 5,6-epoxy-3,3'-dihydroxy-7',8'-didehydro-5,6-dihydro-12',13',20'-trinor-b,b-caroten-19,11-olide
- Semi-a-carotenone 5,6-Seco-b,e-carotene-5,6-dione
- Semi-b-carotenone 5,6-seco-b,b-carotene-5,6-dione or 5',6'-seco-b,b-carotene-5',6'-dione
- Triphasiaxanthin 3-Hydroxysemi-b-carotenone 3'-Hydroxy-5,6-seco-b,b-carotene-5,6-dione or 3-hydroxy-5',6'-seco-b,b-carotene-5',6'-dione
- retro Carotenoids and retro Apo Carotenoids
- Eschscholtzxanthin 4',5'-Didehydro-4,5'-retro-b,b-carotene-3,3'-diol
- Eschscholtzxanthone 3'-Hydroxy-4',5'-didehydro-4,5'-retro-b,b-caroten-3-one
- Rhodoxanthin 4',5'-Didehydro-4,5'-retro-b,b-carotene-3,3'-dione
- Tangeraxanthin 3-Hydroxy-5'-methyl-4,5'-retro-5'-apo-b-caroten-5'-one or 3-hydroxy-4,5'-retro-5'-apo-b-caroten-5'-one
- Higher Carotenoids
- Nonaprenoxanthin 2-(4-Hydroxy-3-methyl-2-butenyl)-7',8',11',12'-tetrahydro-e,y-carotene
- Decaprenoxanthin 2,2'-Bis(4-hydroxy-3-methyl-2-butenyl)-e,e-carotene
- C.p. 450 2-[4-Hydroxy-3-(hydroxymethyl)-2-butenyl]-2'-(3-methyl-2-butenyl)-b,b-carotene
- C.p. 473 2'-(4-Hydroxy-3-methyl-2-butenyl)-2-(3-methyl-2-butenyl)-3',4'-didehydro-l',2'-dihydro-b,y-caroten-1'-ol
- Bacterioruberin 2,2'-Bis(3-hydroxy-3-methylbutyl)-3,4,3',4'-tetradehydro-1,2,1',2'-tetrahydro-y,y-carotene-1,1'-dio
ReferencesISBN links support NWE through referral fees
.[7]
- McGraw-Hill Concise Encyclopedia of Science & Technology, 5th edition. 2005. New York: McGraw-Hill. ISBN 0071429573.
- Sims, J., and T. G. Odle. 2005. Carotenoids. In J. L. Longe, The Gale Encyclopedia of Alternative Medicine, Farmington Hills, Mich: Thomson/Gale, ISBN 0787693960
Classifications
Carotenoids can have many classifications. Some are alcohols, hydrocarbons, ethers, epoxides, ketones, acids, etc. They can be classified also into Apo Carotenoids, Nor and Seco Carotenoids, retro Carotenoids, retro Apo Carotenoids and Higher Carotenoids.
See also
- Phytochemistry
- List of phytochemicals and foods in which they are prominent
External links
- http://www.carotenoidsociety.org/
- Carotenoid Terpenoids
- Carotenoids as Flavor and Fragrance Precursors
- MeSH Carotenoids
| ||||||||||||||
| ||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Bjelakovic G, et al (2007). Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297 (8): 842–57.
- ↑ It is known that taking beta-carotene supplements is harmful for smokers, and the meta-analysis of Bjelakovic et al. was influenced by inclusion of these studies. See the letter to JAMA by Philip Taylor and Sanford Dawsey and the reply by the authors of the original paper.
- ↑ Unlu N, et al (2005). Carotenoid Absorption from Salad and Salsa by Humans Is Enhanced by the Addition of Avocado or Avocado Oil. Human Nutrition and Metabolism 135 (3): 431–6.
- ↑ Choo Yuen May Palm oil carotenoids
- ↑ Alija AJ, Bresgen N, Sommerburg O, Siems W, Eckl PM (2004). Cytotoxic and genotoxic effects of {beta}-carotene breakdown products on primary rat hepatocytes. Carcinogenesis 25 (5): 827–31.
- ↑ Liu GY, Essex A, Buchanan JT, et al (2005). Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 202 (2): 209–15.
- ↑ Armstrong GA, Hearst JE (1996). Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 10 (2): 228–37.}