Difference between revisions of "Adenosine triphosphate" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 19: | Line 19: | ||
| [[Molecular mass]] | | [[Molecular mass]] | ||
| 507.181 g mol<sup>-1</sup> | | 507.181 g mol<sup>-1</sup> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| [[CAS registry number|CAS number]] | | [[CAS registry number|CAS number]] | ||
| 56-65-5 | | 56-65-5 | ||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
|} | |} | ||
| Line 40: | Line 27: | ||
<!-- TEXT —> | <!-- TEXT —> | ||
| − | '''Adenosine triphosphate''' ('''ATP''') is the chemical compound known in [[biochemistry]] as the "[[molecule|molecular]] currency" of intracellular [[energy]] transfer; that is, ATP is able to store and transport chemical energy within [[cell (biology)|cell]]s. All cells& | + | '''Adenosine triphosphate''' ('''ATP''') is the chemical compound known in [[biochemistry]] as the "[[molecule|molecular]] currency" of intracellular [[energy]] transfer; that is, ATP is able to store and transport chemical energy within [[cell (biology)|cell]]s. All cells—prokaryote, animal, and plant—use ATP as their principal energy-carrying molecule and as the main energy source for driving energy-requiring reactions (endergonic); as such, ATP also has been referred to as "life's energy currency" (Luria, Gould and Singer 1981). |
| − | In short, living cells require energy to survive and function, and most of this energy comes either via radiant energy | + | In short, living cells require energy to survive and function, and most of this energy comes either via radiant energy or from chemical energy tied up in interatomic bonds of nutrient molecules. When nutrient molecules derived from carbohydrates, fats, and proteins are oxidized by cells, a portion of the free energy released can be captured in the chemical bonds of ATP. ATP allows cells to store energy as chemical potential and to circulate and use this energy. |
In addition to its energy-related function, ATP also plays an important role in the synthesis of [[nucleic acid]]s. In signal transduction pathways, ATP is used to provide the phosphate for the protein-kinase reactions. | In addition to its energy-related function, ATP also plays an important role in the synthesis of [[nucleic acid]]s. In signal transduction pathways, ATP is used to provide the phosphate for the protein-kinase reactions. | ||
| − | The ubiqutous presence of ATP in the cells of all living organisms indicates that it must have appeared early in the history of cellular life. The intricacy of how ATP is used by the body in various metabolic pathways, and its fundamental importance to living systems, reveals the complex | + | The ubiqutous presence of ATP in the cells of all living organisms indicates that it must have appeared early in the history of cellular life. The intricacy of how ATP is used by the body in various metabolic pathways, and its fundamental importance to living systems, reveals the complex coordination required between parts of living systems. |
==Chemical properties== | ==Chemical properties== | ||
| − | ATP consists of | + | ATP consists of adenosine and three attached [[phosphate]] groups(triphosphate). Adenosine is composed to two major molecular entities, [[adenine]] (a nitrogen-contaning molecule) and ribose (a five-carbon sugar). [[Adenosine monophosphate]] (AMP) has one attached phosphate group, and [[Adenosine diphosphate]] (ADP) has two attached phosphate groups. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{| | {| | ||
| Line 69: | Line 46: | ||
|} | |} | ||
| − | The | + | The three linked phosphoryl groups, starting with that on AMP, are referred to as the alpha (α), beta (β), and gamma (γ) phosphates. These linked phosphate groups are the "business end" of the molecule, as ATP stores energy in the bonds between the phosphate groups. A molecule of ATP is sometimes written as A~P~P~P, with the "~" representing a bond that contains potential chemical energy. |
| − | + | ATP is extremely rich in chemical energy, in particular between the second and third phosphate groups. As these chemical bonds are broken (as ATP is converted into ADP) energy is provided for cellular functions. The net change in energy of the decomposition of ATP into [[ADP]] and an inorganic phosphate is -12 kCal / mole ''in vivo'', or inside of a living cell, and -7.3 kCal / mole ''in vitro'', or in laboratory conditions. This massive release in energy makes the decomposition of ATP extremely good at releasing energy (exergonic), and hence useful as a means for chemically storing energy, and being released to help with reactions requiring a net input of energy (endergonic). | |
| − | ATP energy | ||
| − | == | + | ==Synthesis== |
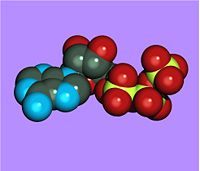
| − | + | [[Image:Atp_space_filling_ray_trace.jpg|200px|thumb|Space filling image of ATP]] | |
| + | ATP can be produced by various cellular processes: Under aerobic conditions, the synthesis occurs in [[mitochondria]] during [[oxidative phosphorylation]] and is catalyzed by ATP synthase; to a lesser degree, under anaerobic conditions, this is done through substrate phosphorylation catalyzed by two enzymes: phosphoglycerate kinase (PGK) and pyruvate kinase. | ||
| − | + | ATP is also synthesized through several so-called "replenishment" reactions catalyzed by the enzyme families of NDKs (nucleoside diphosphate kinases), which use other nucleoside triphosphates as a high-energy phosphate donor, and the ATP:guanido-phosphotransferase family, which uses [[creatine]]. | |
| − | |||
| − | + | ::[[adenosine diphosphate|ADP]] + [[guanosine triphosphate|GTP]] <math>\to</math> ATP + [[guanosine diphosphate|GDP]] | |
| − | + | In plants, ATP is synthesized in [[chloroplast]]s by [[photosynthesis]] during the light reactions of [[photosynthesis]]. However, this ATP is then used to power the [[Calvin cycle]] step of photosynthesis and so photosynthesis does not result in an overall production of ATP. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | The main fuels for ATP synthesis are [[glucose]] and [[fatty acid]]s. First, glucose is broken down into pyruvate in the cytosol. Two molecules of ATP are generated for each molecule of glucose. The terminal stages of ATP synthesis are carried out in the mitochondrion and can generate up to 36 ATP, which is 40% energy efficiency. (See [[citric acid cycle]]) | |
| − | + | ==Function== | |
| + | ATP energy is released when hydrolysis of the high energy phosphate-phosphate]] bonds is carried out. This energy can be used by a variety of [[enzyme]]s, motor [[protein]]s, and transport proteins to carry out the work of the cell. Also, the hydrolysis yields free inorganic phosphate (P<sub>i</sub>) and [[adenosine diphosphate|ADP]], which can be broken down further to another P<sub>i</sub> and [[adenosine monophosphate|AMP]]. ATP can also be broken down to AMP directly, with the formation of pyrophosphate (PP<sub>i</sub>). This last reaction has the advantage of being an effectively irreversible process in aqueous solution. | ||
| − | + | ==ATP in the human body== | |
| − | + | The total quantity of ATP in the human body at any one time is about 0.1 [[Mole (unit)|mole]]. Yet, adults convert daily a quantity of ATP corresponding to at least half his or her body weight, and nearly a ton during a day of hard work. That is, the energy used by human cells requires the [[hydrolysis]] of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2000 to 3000 times during a single day. There is limited capacity to store ATP in a cell, and it is depleted in seconds, hence its consumption must closely follow its synthesis. That is, cells need to continually replenish or resynthesize ATP. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[ | ||
| − | |||
| − | [[ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 22:56, 28 January 2006
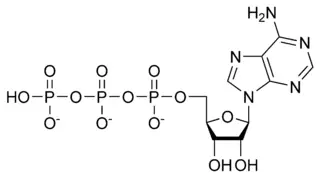
| Adenosine 5'-triphosphate | |
|---|---|
| |
| Chemical name |
[[[5-(6-aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl] methoxy-hydroxy-phosphoryl] oxy-hydroxy-phosphoryl] oxyphosphonic acid |
| Abbreviations | ATP |
| Chemical formula | C10H16N5O13P3 |
| Molecular mass | 507.181 g mol-1 |
| CAS number | 56-65-5 |
Adenosine triphosphate (ATP) is the chemical compound known in biochemistry as the "molecular currency" of intracellular energy transfer; that is, ATP is able to store and transport chemical energy within cells. All cells—prokaryote, animal, and plant—use ATP as their principal energy-carrying molecule and as the main energy source for driving energy-requiring reactions (endergonic); as such, ATP also has been referred to as "life's energy currency" (Luria, Gould and Singer 1981).
In short, living cells require energy to survive and function, and most of this energy comes either via radiant energy or from chemical energy tied up in interatomic bonds of nutrient molecules. When nutrient molecules derived from carbohydrates, fats, and proteins are oxidized by cells, a portion of the free energy released can be captured in the chemical bonds of ATP. ATP allows cells to store energy as chemical potential and to circulate and use this energy.
In addition to its energy-related function, ATP also plays an important role in the synthesis of nucleic acids. In signal transduction pathways, ATP is used to provide the phosphate for the protein-kinase reactions.
The ubiqutous presence of ATP in the cells of all living organisms indicates that it must have appeared early in the history of cellular life. The intricacy of how ATP is used by the body in various metabolic pathways, and its fundamental importance to living systems, reveals the complex coordination required between parts of living systems.
Chemical properties
ATP consists of adenosine and three attached phosphate groups(triphosphate). Adenosine is composed to two major molecular entities, adenine (a nitrogen-contaning molecule) and ribose (a five-carbon sugar). Adenosine monophosphate (AMP) has one attached phosphate group, and Adenosine diphosphate (ADP) has two attached phosphate groups.
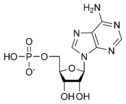 Adenosine monophosphate AMP |
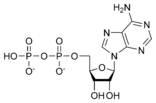 Adenosine diphosphate ADP |
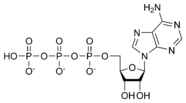 Adenosine triphosphate ATP |
The three linked phosphoryl groups, starting with that on AMP, are referred to as the alpha (α), beta (β), and gamma (γ) phosphates. These linked phosphate groups are the "business end" of the molecule, as ATP stores energy in the bonds between the phosphate groups. A molecule of ATP is sometimes written as A~P~P~P, with the "~" representing a bond that contains potential chemical energy.
ATP is extremely rich in chemical energy, in particular between the second and third phosphate groups. As these chemical bonds are broken (as ATP is converted into ADP) energy is provided for cellular functions. The net change in energy of the decomposition of ATP into ADP and an inorganic phosphate is -12 kCal / mole in vivo, or inside of a living cell, and -7.3 kCal / mole in vitro, or in laboratory conditions. This massive release in energy makes the decomposition of ATP extremely good at releasing energy (exergonic), and hence useful as a means for chemically storing energy, and being released to help with reactions requiring a net input of energy (endergonic).
Synthesis
ATP can be produced by various cellular processes: Under aerobic conditions, the synthesis occurs in mitochondria during oxidative phosphorylation and is catalyzed by ATP synthase; to a lesser degree, under anaerobic conditions, this is done through substrate phosphorylation catalyzed by two enzymes: phosphoglycerate kinase (PGK) and pyruvate kinase.
ATP is also synthesized through several so-called "replenishment" reactions catalyzed by the enzyme families of NDKs (nucleoside diphosphate kinases), which use other nucleoside triphosphates as a high-energy phosphate donor, and the ATP:guanido-phosphotransferase family, which uses creatine.
- ADP + GTP ATP + GDP
In plants, ATP is synthesized in chloroplasts by photosynthesis during the light reactions of photosynthesis. However, this ATP is then used to power the Calvin cycle step of photosynthesis and so photosynthesis does not result in an overall production of ATP.
The main fuels for ATP synthesis are glucose and fatty acids. First, glucose is broken down into pyruvate in the cytosol. Two molecules of ATP are generated for each molecule of glucose. The terminal stages of ATP synthesis are carried out in the mitochondrion and can generate up to 36 ATP, which is 40% energy efficiency. (See citric acid cycle)
Function
ATP energy is released when hydrolysis of the high energy phosphate-phosphate]] bonds is carried out. This energy can be used by a variety of enzymes, motor proteins, and transport proteins to carry out the work of the cell. Also, the hydrolysis yields free inorganic phosphate (Pi) and ADP, which can be broken down further to another Pi and AMP. ATP can also be broken down to AMP directly, with the formation of pyrophosphate (PPi). This last reaction has the advantage of being an effectively irreversible process in aqueous solution.
ATP in the human body
The total quantity of ATP in the human body at any one time is about 0.1 mole. Yet, adults convert daily a quantity of ATP corresponding to at least half his or her body weight, and nearly a ton during a day of hard work. That is, the energy used by human cells requires the hydrolysis of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2000 to 3000 times during a single day. There is limited capacity to store ATP in a cell, and it is depleted in seconds, hence its consumption must closely follow its synthesis. That is, cells need to continually replenish or resynthesize ATP.