Thyroid
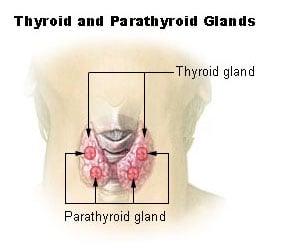
The thyroid (from the Greek word for "shield," after its shape) is one of the larger endocrine glands in the body. It is a double-lobed structure located in the neck and produces hormones, principally thyroxine (T4) and triiodothyronine (T3), that regulate the rate of metabolism and affect the growth and rate of function of many other systems in the body. The hormone calcitonin is also produced and controls calcium blood levels. Iodine is necessary for the production of both hormones. Hyperthyroidism (overactive thyroid) and hypothyroidism (underactive thyroid) are the most common problems of the thyroid gland.
Anatomy
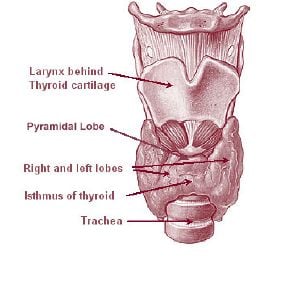
The thyroid is situated on the front side of the neck, starting at the oblique line on the thyroid cartilage (just below the laryngeal prominence or Adam's apple), and extending to the 6th Tracheal ring (C-shaped cartilagenous ring of the trachea). Vertibral levels are inappropriate to demarkate the glands upper and lower border with vertebral levels as it moves position in relation to these during swallowing. The thyroid lies over the trachea and is covered by layers of pretracheal fascia (allowing for movement), muscle, and skin.
The thyroid is one of the larger endocrine glands - 10-20 grams in adults and butterfly-shaped. The wings correspond to the lobes and the body to the isthmus of the thyroid. It may enlarge substantially during pregnancy and when affected by a variety of diseases.
Blood supply
The thyroid gland is supplied by two pairs of arteries: the superior and inferior thyroid arteries of each side. The superior thyroid artery is the first branch of the external carotid artery and supplies mostly the upper half of the thyroid gland, while the inferior thyroid artery is the major branch of the thyrocervical trunk, which comes off of the subclavian artery. In 10% of people, there is an additional thyroid artery, the thyreoidea ima, that arises from the brachiocephalic trunk or the arch of the aorta. Lymph drainage follows the arterial supply.
There are three main veins that drain the thyroid to the superior vena cava (which opens directly into the right atrium of the heart): the superior, middle and inferior thyroid veins.
In comparison to the other organs of the body, the thyroid gland receives one of the largest blood supplies per gram weight. The largest blood supply is seen in the carotid arch baroreceptor organ (regulates variations in blood pressure due to changes in posture).
Embryologic development
In the fetus, at 3-4 weeks of gestation, the thyroid gland appears as an epithelial proliferation in the floor of the pharynx at the base of the tongue between the tuberculum impar and the copula at a point latter indicated by the foramen cecum. Subsequently the thyroid descends in front of the pharyngeal gut as a bilobed diverticulum through the thyroglossal duct. Over the next few weeks, it migrates to the base of the neck. During migration, the thyroid remains connected to the tongue by a narrow canal, the thyroglossal duct.
Follicles of the thyroid begin to make colloid in the 11th week and thyroxine by the 18th week.
Histology of the thyroid
The thyroid is composed of spherical follicles that selectively absorb iodine (as iodide ions, I-) from the blood for production of thyroid hormones. Twenty-five percent of all the body's iodide ions are in the thyroid gland. The follicles are made of a single layer of thyroid epithelial cells, which secrete T3 and T4. Inside the follicles is a colloid which is rich in a protein called thyroglobulin. The colloidal material serves as a reservoir of materials for thyroid hormone production and, to a lesser extent, a reservoir of the hormones themselves, as it binds to it. Scattered among follicular cells and in spaces between the spherical follicles are another type of thyroid cell, parafollicular cells or C cells, which secrete calcitonin.
Physiology
The primary function of the thyroid is production of the hormones thyroxine (T4), triiodothyronine (T3), and calcitonin. Up to 40% of the T4 is converted to T3 by peripheral organs such as the liver, kidney and spleen. T3 is about ten times more active than T4[1].
T3 and T4 production and action
Thyroxine is synthesized by the follicular cells from free tyrosine and on the tyrosine residues of the protein called thyroglobulin (TG). Iodine, which is captured with the "iodine trap" by the hydrogen peroxide generated by the enzyme thyroid peroxidase (TPO),[2] is linked to the 3' and 5' sites of the benzene ring of the tyrosine residues on TG and on free tyrosine (amino acid). Upon stimulation by TSH (see below), the follicular cells reabsorb TG and proteolytically cleave the iodinated tyrosines from TG, forming T4 and T3 (in T3, one iodine is absent compared to T4). These are then released into the blood. Deiodinase enzymes convert T4 to T3[3]. Thyroid hormone that is secreted from the gland is about 90% T4 and about 10% T3[1].
Cells of the brain are a major target for thyroid hormone. Thyroid hormones play a particularly crucial role in brain development during pregnancy[4]. A transport protein (OATP1C1) has been identified that seems to be important for T4 transport across the blood brain barrier[5]. A second transport protein (MCT8) is important for T3 transport across brain cell membranes[5].
In the blood, T4 and T3 are partially bound to thyroxine-binding globulin, transthyretin and albumin. Only a very small fraction of the circulating hormone is free (unbound) - T4 0.03% and T3 0.3%. Soley, the free fraction has hormonal activity. As with the steroid hormones and retinoic acid, thyroid hormones cross the cell membrane and bind to intracellular receptors (α1, α2, β1 and β2), which act alone, in pairs or together with the retinoid X-receptor as transcription factors to modulate DNA transcription[1].
T3 and T4 regulation
The production of thyroxine is regulated by thyroid-stimulating hormone (TSH), rwhich is eleased by the anterior pituitary. The thyroid and thyrotropes form a negative feedback loop: TSH production is suppressed when the T4 levels are high, and vice versa. The TSH production itself is modulated by thyrotropin-releasing hormone, which is produced by the hypothalamus and secreted at an increased rate in situations such as cold (in which an accelerated metabolism would generate more heat). TSH production is blunted by somatostatin, rising levels of glucocorticoids and sex hormones (estrogen and testosterone), and excessively high blood iodide concentration.
Calcitonin
An additional hormone produced by the thyroid contributes to the regulation of blood calcium levels. Parafollicular cells produce calcitonin in response to hypercalcemia (high levels of calcium). Calcitonin stimulates movement of calcium into bone, in opposition to the effects of parathyroid hormone (PTH). However, calcitonin seems far less essential than PTH, as calcium metabolism remains clinically normal after removal of the thyroid, but not the parathyroids.
It may be used diagnostically as a tumor marker for a form of thyroid cancer (medullary thyroid adenocarcinoma), in which high calcitonin levels may be present and elevated levels after surgery may indicate recurrence. It may even be used on biopsy samples from suspicious lesions (e.g. swollen lymph nodes) to establish whether they are metastasis of the original cancer.
Calcitonin can be used therapeutically for the treatment of hypercalcemia or osteoporosis.
The significance of iodine
In areas of the world where iodine (essential for the production of thyroxine, which contains four iodine atoms) is lacking in the diet, the thyroid gland can be considerably enlarged, resulting in the swollen necks of endemic goiter.
Thyroxine is critical to the regulation of metabolism and growth throughout the animal kingdom. Among amphibians, for example, administering a thyroid-blocking agent such as propylthiouracil (PTU) can prevent tadpoles from metamorphosing into frogs; conversely, administering thyroxine will trigger metamorphosis.
In humans, children born with thyroid hormone deficiency will have physical growth and development problems, and brain development can also be severely impaired, in the condition referred to as cretinism (defined by physical deformity, dwarfism, mental retardation, and often by goiter). Newborn children in many developed countries are now routinely tested for thyroid hormone deficiency as part of newborn screening by analysis of a drop of blood. Children with thyroid hormone deficiency are treated by supplementation with levothyroxine (synthetic thyroxine), which enables them to grow and develop normally.
Because of the thyroid's selective uptake and concentration of what is a fairly rare element, it is sensitive to the effects of various radioactive isotopes of iodine produced by nuclear fission. In the event of large accidental releases of such material into the environment, the uptake of radioactive iodine isotopes by the thyroid can, in theory, be blocked by saturating the uptake mechanism with a large surplus of non-radioactive iodine, taken in the form of potassium iodide tablets. While biological researchers making compounds labelled with iodine isotopes do this, in the wider world such preventive measures are usually not stockpiled before an accident, nor are they distributed adequately afterward. One consequence of the Chernobyl disaster (1986 nuclear power accident in USSR) was an increase in thyroid cancers in children in the years following the accident. [2]
The use of iodized salt is an efficient way to add iodine to the diet. It has eliminated endemic cretinism in most developed countries, and some governments have made the iodination of flour or salt mandatory. Potassium iodide and sodium iodide are the most active forms of supplemental iodine.
Diseases of the thyroid gland
Hyper- and hypofunction (affects about 2% of the population):
Hypothryoidism is a condition marked by decreased activity of the gland. This can occur pathologically in the body (see example of some disease states below) as well as after removal of thyroid gland following surgery for cancer or even hyperfunction. This condition is commonly marked by weight gain, heat intolerance, lethargy, constipation, hair loss, skin changes, cardiac problems, etc. The main stay or treatment involves daily thyroid hormone replacement (thyroxine) and subsequent thyroid stimulating hormone (TSH) level monitoring.
Hyperthyroidism is a disease state marked by excessive function of the thyroid gland. This can occur pathologically with no visible physical changes to the thryoid gland or with changes (some disease states are given below). It is characteristically marked by a slew of symptoms, most commonly excessive sweating, weight loss, diarrhea, palpitations, proximal muscle weakness, neurological changes, etc. Treatment for this condition is most commonly removal of the gland or its destruction with iodine compounds, followed by daily thyroxine hormone replacement therapy.
It is always imperative to remember that thyroid function is unrelated to the size of the thyroid gland.
- Hypothyroidism (underactivity)
- Hashimoto's thyroiditis
- Ord's thyroiditis
- Postoperative hypothyroidism
- Postpartum thyroiditis
- Silent thyroiditis
- Acute thyroiditis
- Iatrogenic hypothyroidism
- Hyperthyroidism (overactivity)
- Thyroid storm
- Graves-Basedow disease
- Toxic thyroid nodule
- Toxic nodular struma (Plummer's disease)
- Hashitoxicosis
- Iatrogenic hyperthyroidism
- De Quervain thyroiditis (inflammation starting as hyperthyroidism, can end as hypothyroidism)
Anatomical problems:
- Goiter (enlargement of the thyroid gland)
- Endemic goiter
- Diffuse goiter
- Multinodular goiter
- Lingual thyroid
- Thryoglossal duct cyst
Tumors:
- Thyroid adenoma
- Thyroid cancer
- Papillary
- Follicular
- Medullary
- Anaplastic
- Lymphomas and metastasis from elsewhere (rare)
Deficiencies:
- Cretinism
Medication linked to thyroid disease includes amiodarone, lithium salts, some types of interferon and aldesleukin (IL-2).
Diagnosis
The measurement of thyroid-stimulating hormone (TSH) levels is often used by doctors as a screening test. Elevated TSH levels can signify an inadequate hormone production, while suppressed levels can point at excessive unregulated production of hormone. If TSH is abnormal, decreased levels of thyroid hormones T4 and T3 may be present; these may be determined to confirm this. Autoantibodies may be detected in various disease states (anti-TG, anti-TPO, TSH receptor stimulating antibodies). There are two cancer markers for thyroid derived cancers. Thyroglobulin (TG) for well differentiated papillary or follcular adenocarcinoma, and the rare medullary thyroid cancer has calcitonin as the marker. Very infrequently, thyroxine-binding globulin (TBG) and transthyretin levels may be abnormal; these are not routinely tested.
Nodules of the thyroid may require medical ultrasonography to establish their nature. The main characteristics of a thyroid nodule on high frequency thyroid ultrasound that suggest possible cancer are:
- 1. irregular border
- 2. hypoechoic (less echogenic than the surrounding tissue)
- 3. microcalcifications
- 4. taller than wide shape on transverse study
- 5. significant intranodular blood flow by power Doppler.
Benign characteristics include:
- 1. hyperechoic
- 2. smooth borders
- 3. "comet tail" artifact as sound waves bounce off intranodular colloid; however, these criteria alone can help select nodules for biopsy, but no criteria is 100%.
The ideal way to assure that a nodule is not cancerous is a biopsy. To be sure you have sampled the specific nodule of interest, even if you can not feel it, ultrasound guided fine needle aspiration is recommended. Free hand fine needle aspiration (FNA) may be performed, on palpable nodules, but has a higher error rate, or inadequate sample result. If a result is not conclusive, thyroid scintigraphy with iodine-123 may reveal whether the nodule is abnormally active "hot" or inactive "cold". Hot nodules are very, very rarely cancerous; therefore, the endocrinologist may not need to repeat the biopsy. However if it is not hot, an inconclusive FNA result may warrant a repeat biopsy, but this time, not by free hand, but by ultrasound guided FNA technique.
Hashimoto's thyroiditis may be the background disease for a rapidly growing mass caused by a rare case of thyroid lymphoma. The rapid growth in a previous long standing stable thyroiditis should be biopsied by ultrasound guided needle, and live cells collected in special medium for flow cytometry. The exact type of lymphoma can be determined by FNA, without surgery.
Treatment
Medical treatment
Levothyroxine is a stereoisomer of thyroxine which is degraded much slower and can be administered once daily in patients with hypothyroidism. Stereoisomers are molecules whose atomic connectivity is the same but atomic arrangement in space is different.
Graves' disease may be treated with the thioamide drugs propylthiouracil, carbimazole, methimazole, or rarely with Lugol's solution. Hyperthyroidism as well as thyroid tumors may be treated with radioactive iodine.
Percutaneous Ethanol Injections, PEI, for therapy of recurrent thyroid cysts, and metastatic thyroid cancer lymph nodes, as an alternative to the usual surgical method.
Thyroid surgery
Thyroid surgery is performed for a variety of reasons. A nodule, or lobe, of the thyroid is sometimes removed for biopsy or for the presence of an autonomously functioning thyroid adenoma causing hyperthyroidism. A large majority of the thyroid may be removed, a subtotal thyroidectomy, to treat the hyperthyroidism of Graves' disease, or to remove a goitre that is unsightly or impinges on vital structures. A complete thyroidectomy of the entire thyroid, including associated lymph nodes, is the preferred treatment for thyroid cancer. Removal of the bulk of the thyroid gland usually produces hypothyroidism, unless the person takes thyroid hormone replacement.
If the thyroid gland must be removed surgically, care must be taken to avoid damage to the adjacent structures of the parathyroid glands and the recurrent laryngeal nerve. Both are susceptible to accidental removal and/or injury during thyroid surgery. The parathyroid glands produce parathyroid hormone (PTH), a hormone needed to maintain adequate amounts of calcium in the blood. Removal results in hypoparathyroidism and a need for supplemental calcium and vitamin D each day. The recurrent laryngeal nerves, which run along the posterior thyroid, provide motor control for all external muscles of the larynx, except for the cricothyroid muscle. Accidental laceration of either of the two or both recurrent laryngeal nerves may cause paralysis of the vocal cords and their associated muscles, changing voice quality.
Radioiodine therapy
Large goiters that cause symptoms, but do not harbor cancer, after evaluation, and biopsy of suspicious nodules can be treated by an alternative therapy with radioiodine. The iodine uptake can be high in countries with iodine deficiency, but low in iodine sufficient countries. The 1999 release of rhTSH thyrogen in the USA, can boost the uptakes to 50-60% allowing the therapy with iodine 131. The gland shrinks by 50-60%, but can cause hypothyroidism, and rarely pain syndrome cause by radiation thyroiditis that is short lived and treated by steroids. Rare cases of Graves' disease have been reported after goiter I/131 therapy. This is still an off label use of Thyrogen, but is a very excellent alternative to surgery.
History
The thyroid was first identified by the anatomist Thomas Wharton (whose name is also eponymized in Wharton's duct of the submandibular gland) in 1656. Thyroid hormone (or thyroxin) was identified in the 19th century.
ReferencesISBN links support NWE through referral fees
- ↑ 1.0 1.1 The thyroid gland in Endocrinology: An Integrated Approach by Stephen Nussey and Saffron Whitehead (2001) Published by BIOS Scientific Publishers Ltd. ISBN 1-85996-252-1 .
- ↑ Ekholm R, Bjorkman U (1997). Glutathione peroxidase degrades intracellular hydrogen peroxide and thereby inhibits intracellular protein iodination in thyroid epithelium. Endocrinology 138 (7): 2871-2878. PMID 9202230.
- ↑ Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002). Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23 (1): 38-89. PMID 11844744.
- ↑ Kester MH, Martinez de Mena R, Obregon MJ, Marinkovic D, Howatson A, Visser TJ, Hume R, Morreale de Escobar G (2004). Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab 89 (7): 3117-3128. PMID 15240580.
- ↑ 5.0 5.1 Jansen J, Friesema ECH, Milici C, Visser TJ (2005). Thyroid hormone transporters in health and disease. Thyroid 15;757-768. PMID 16131319.
External links
- New Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer- American Thyroid Association Taskforce
- Thyroid Disease Manager (free online textbook)
- Thyroid Disease (Nuclear Medicine Information)
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.