Difference between revisions of "Nitrate" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
Rosie Tanabe (talk | contribs) m |
||
| (16 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Copyedited}}{{Images OK}}{{Submitted}}{{Approved}}{{Paid}} |
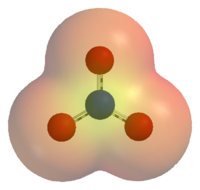
| − | [[Image:Nitrate-ion-elpot.png|thumb|right|200px|An [[electric potential|electrostatic potential]] map of the nitrate ion. | + | [[Image:Nitrate-ion-elpot.png|thumb|right|200px|An [[electric potential|electrostatic potential]] map of the nitrate ion. Areas colored red are lower in energy than areas colored yellow.]] |
| − | + | ||
| − | In [[inorganic chemistry]], a '''nitrate''' is a [[salt]] of [[nitric acid]] | + | In [[inorganic chemistry]], a '''nitrate''' is a [[salt]] of [[nitric acid]] characterized by a negatively charged [[ion]] composed of one [[nitrogen]] [[atom]] bound to three [[oxygen]] atoms. In [[organic chemistry]], the term ''nitrates'' refers to the [[esters]] of nitric acid and various [[alcohols]]. |
| + | |||
| + | Nitrates play significant roles in our lives and in the rest of the natural world. In particular, they form an important source of nitrogen for [[plant]] growth, and therefore for other [[organism]]s that derive their nutrition from plants. We use nitrates for a variety of purposes, including [[fertilizer]]s, [[food preservative]]s, [[medicine]], and [[explosive]]s. | ||
| + | {{toc}} | ||
| + | On the down side, the excessive use of nitrate-containing fertilizers has led to pollution of groundwater and surface waters in various agricultural regions, with adverse effects on aquatic life. In addition, there is concern that ammonium nitrate may be used to make explosives for terrorist activities. | ||
| + | |||
| + | ==Occurrence, history, and production== | ||
| + | [[Image:AYool WOA surf NO3.png|thumb|right|250px|Annual mean sea surface '''nitrate''' for the [[World Ocean]]. Data from the [[World Ocean Atlas]] [http://www.nodc.noaa.gov/OC5/WOA01/ 2001].]] | ||
| + | |||
| + | Solid nitrates are not very abundant in nature as they are very soluble. They can appear where nitrogen-containing groundwater is evaporating (such as in soils of arid regions and on [[animal]] shed walls). [[Nitrification]] [[bacteria]] in the soil are also needed for the process. | ||
| + | |||
| + | The first commercially exploited source was [[India]], providing the [[British Empire]] with a reliable supply. By contrast the [[Europe]]an continental powers had to collect scrapings from walls and barns, install saltpeter farms (based on aging and leaching manure and urine). The chemist [[Lavoisier]] was also a tax collector and commissioner of the Saltpeter Administration. Later, the large deposits of [[#Sodium nitrate|sodium nitrate]] in the [[Atacama Desert]] of northern Chile acquired economic significance. | ||
| + | |||
| + | Until the early part of the twentieth century, there were no known methods for the chemical synthesis of nitrates. Chile was a major exporter, and European countries were dependent on its nitrates for use as fertilizer to feed their people. Nitrates were needed to produce military explosives as well. These two uses influenced world history in significant ways. Had the Germans not devised the [[Haber process|Haber]] and [[Ostwald process|Ostwald]] processes for producing nitrate, they would not have been able to feed their civilian population and armies, nor continued to make explosives. The First World War might have ended as a direct result of embargo of essential raw materials. With the aid of organic chemistry, however, the war continued. Nowadays, most nitrates are produced from [[ammonia]] synthesized from atmospheric nitrogen. | ||
==Chemical properties== | ==Chemical properties== | ||
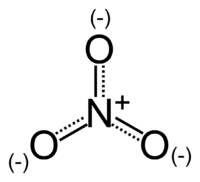
| − | The | + | [[Image:Nitrate-ion.png|thumb|right|200px|The structure and charge of the nitrate ion.]] |
| + | |||
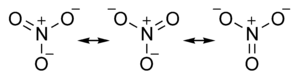
| + | The ''nitrate ion'' is a [[polyatomic ion|polyatomic]] [[ion]] with the [[empirical formula]] [[nitrogen|N]][[oxygen|O]]<sub>3</sub><sup>−</sup> and a [[molecular mass]] of 62.0049. It consists of one central nitrogen [[atom]] surrounded by three identical [[oxygen]] atoms in a [[trigonal planar]] arrangement. The nitrate ion, which carries a [[formal charge]] of −1, can be represented as a "hybrid" of the following three [[resonance structures]]: | ||
[[Image:Nitrate ion resonance structures.png|center|300px]] | [[Image:Nitrate ion resonance structures.png|center|300px]] | ||
| − | The nitrate ion is the [[conjugate acid|conjugate base]] of nitric acid. A nitrate salt forms when a positively charged ion attaches to the negatively charged oxygen atoms of the ion, forming an ionic [[chemical compound|compound]]. Almost all nitrates are [[solubility|soluble]] in [[water]] at [[standard temperature and pressure]]. | + | The structure may also be represented in the form of the diagram on the right. |
| + | |||
| + | The nitrate ion is the [[conjugate acid|conjugate base]] of nitric acid. A nitrate salt forms when a positively charged ion (such as a metal ion) attaches to the negatively charged oxygen atoms of the ion, forming an ionic [[chemical compound|compound]]. Almost all nitrates are [[solubility|soluble]] in [[water]] at [[standard temperature and pressure]]. | ||
| + | |||
| + | In [[organic chemistry]], a nitrate is a [[functional group]] with the general chemical formula RONO<sub>2</sub>, where R stands for any organic residue. These nitrates are the [[ester]]s of nitric acid and [[alcohol]]s, formed by the process known as ''nitroxylation''. Examples are: | ||
| + | * methyl nitrate, formed by reaction of [[methanol]] and nitric acid<ref>Black, Alvin P., and Frank H. Babers. "Methyl Nitrate." ''Organic Syntheses'' 2 (1943): 412; 19 (1939): 64. [http://www.orgsynth.org/orgsyn/pdfs/CV2P0412.pdf Methyl Nitrate] Retrieved September 13, 2007.</ref> | ||
| + | * the nitrate of [[tartaric acid]]<ref>Snyder, H.R., et al., "Imidazole." ''Organic Syntheses'' 3 (1955): 471; 22 (1942): 65. [http://www.orgsynth.org/orgsyn/pdfs/CV3P0471.pdf Imidazole] Retrieved September 13, 2007.</ref> | ||
| + | * [[nitroglycerin]]. | ||
| + | |||
| + | == Effects on aquatic life == | ||
| + | |||
| + | In [[freshwater]] or [[estuary|estuarine]] systems close to land, nitrate concentrations can reach high levels, potentially causing the death of [[fish]]. Although the nitrate ion is much less toxic than ammonia or nitrite, levels over 30 parts per million (ppm) of nitrate can inhibit growth, impair the immune system, and cause stress in some aquatic species. | ||
| + | |||
| + | In most cases, high nitrate concentrations in aquatic systems are the result of [[surface runoff]] from agricultural or [[landscape]]d areas that have received excess nitrate fertilizer. These levels of nitrate can also lead to algal blooms, and when nutrients (such as [[potassium]], [[phosphate]], or nitrate) become limiting, [[eutrophication]] can occur. Besides leading to water [[anoxia]], these blooms may cause other changes to [[ecosystem]] functions, favoring some groups of organisms over others. Consequently, as nitrates form a component of [[total dissolved solids]], they are widely used as indicators of [[water quality]]. | ||
| + | |||
| + | == Specific nitrates == | ||
| + | === Ammonium nitrate === | ||
| + | |||
| + | ''Ammonium nitrate'' (NH<sub>4</sub>NO<sub>3</sub>) is commonly used in [[agriculture]] as a high-nitrogen [[fertilizer]]. It can also be used as an [[oxidizing agent]] in [[explosive]]s, especially [[improvised explosive device]]s. | ||
| + | |||
| + | === Potassium nitrate === | ||
| − | + | ''Potassium nitrate'' ([[Potassium|K]][[Nitrogen|N]][[Oxygen|O]]<sub>3</sub>) is a naturally occurring mineral source of nitrogen. Its common names include ''saltpeter'' (''saltpetre''), ''nitrate of potash'', and ''nitre''. It is used in the production of [[nitric acid]], model rocket propellants, and several types of [[fireworks]]. In addition, it is a [[fertilizer]] and [[food preservative]]. Although also used in gunpowder, it is not combustible or flammable by itself. | |
| − | == | + | === Sodium nitrate === |
| − | |||
| − | |||
| − | + | ''Sodium nitrate'' (NaNO<sub>3</sub>) is a type of [[salt]] that has long been used as an ingredient in explosives and solid rocket propellants, in glass and pottery enamel, and as a food preservative (such as in [[hot dog]]s), and has been mined extensively for these purposes. It is also variously known as ''[[caliche (Mineral)|caliche]]'', ''Chile saltpeter'', ''saltpeter'', and ''soda niter''. Chile has the largest reserves of caliche. It can also be manufactured synthetically. | |
| − | |||
| + | === Silver nitrate === | ||
| + | |||
| + | ''Silver nitrate'' ([[silver|Ag]][[Nitrogen|N]][[Oxygen|O]]<sub>3</sub>) is a soluble salt of silver and a corrosive compound. It produces a gray or black stain on skin. As a light-sensitive material, it is used in preparing [[photographic film]]. It is also used in making silver-based explosives and in staining biological samples for research. In addition, it has been used in medicine for its antiseptic properties. | ||
==Related materials== | ==Related materials== | ||
| − | |||
| − | + | * Nitrates should not be confused with [[nitrite]]s, the salts of [[nitrous acid]]. | |
| − | + | * [[Organic compound]]s containing the nitro (NO<sub>2</sub>) [[functional group]] are known as [[nitro compound]]s. | |
==See also== | ==See also== | ||
| − | |||
| − | |||
| − | + | * [[Ammonia]] | |
| − | * [ | + | * [[Fertilizer]] |
| − | * [ | + | * [[Nitric acid]] |
| + | * [[Nitrite]] | ||
| + | * [[Nitrogen]] | ||
| + | * [[Oxygen]] | ||
| + | |||
| + | == Notes == | ||
| + | <references/> | ||
==References== | ==References== | ||
| − | |||
| − | + | * Addiscott, T.M., A.J. Gold, C.A. Oviatt, N. Benjamin, and K.E. Giller. ''Nitrate, Agriculture and the Environment''. CABI Publishing, 2005. ISBN 0851999131 | |
| − | [[Category: | + | * Chang, Raymond. ''Chemistry''. 9th ed. New York: McGraw-Hill Science/Engineering/Math, 2006. ISBN 0073221031 |
| − | [[Category: | + | * Cotton, F. Albert, and Geoffrey Wilkinson. ''Advanced Inorganic Chemistry''. 4th ed. New York: Wiley, 1980. ISBN 0-471-02775-8 |
| − | [[Category: | + | * Razowska-Jaworek, Lidia, and Andrzej Sadurski. ''Nitrates in Groundwater''. International Association of Hydrogeologists Selected Papers. Leiden, the Netherlands: A.A. Balkema, 2005. ISBN 9058096645 |
| − | [[Category: | + | |
| − | [[Category: | + | [[Category:Physical sciences]] |
| + | [[Category:Chemistry]] | ||
| + | [[Category:Organic chemistry]] | ||
| + | [[Category:Inorganic chemistry]] | ||
| + | [[Category:Environmental science]] | ||
| − | + | {{credits|Nitrate|113659405|Ammonium_nitrate|116191451|Potassium_nitrate|116963326|Sodium_nitrate|112800697|Silver_nitrate|116945875}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 17:10, 15 January 2015
In inorganic chemistry, a nitrate is a salt of nitric acid characterized by a negatively charged ion composed of one nitrogen atom bound to three oxygen atoms. In organic chemistry, the term nitrates refers to the esters of nitric acid and various alcohols.
Nitrates play significant roles in our lives and in the rest of the natural world. In particular, they form an important source of nitrogen for plant growth, and therefore for other organisms that derive their nutrition from plants. We use nitrates for a variety of purposes, including fertilizers, food preservatives, medicine, and explosives.
On the down side, the excessive use of nitrate-containing fertilizers has led to pollution of groundwater and surface waters in various agricultural regions, with adverse effects on aquatic life. In addition, there is concern that ammonium nitrate may be used to make explosives for terrorist activities.
Occurrence, history, and production
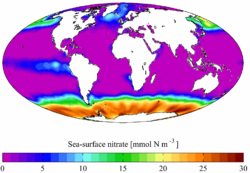
Solid nitrates are not very abundant in nature as they are very soluble. They can appear where nitrogen-containing groundwater is evaporating (such as in soils of arid regions and on animal shed walls). Nitrification bacteria in the soil are also needed for the process.
The first commercially exploited source was India, providing the British Empire with a reliable supply. By contrast the European continental powers had to collect scrapings from walls and barns, install saltpeter farms (based on aging and leaching manure and urine). The chemist Lavoisier was also a tax collector and commissioner of the Saltpeter Administration. Later, the large deposits of sodium nitrate in the Atacama Desert of northern Chile acquired economic significance.
Until the early part of the twentieth century, there were no known methods for the chemical synthesis of nitrates. Chile was a major exporter, and European countries were dependent on its nitrates for use as fertilizer to feed their people. Nitrates were needed to produce military explosives as well. These two uses influenced world history in significant ways. Had the Germans not devised the Haber and Ostwald processes for producing nitrate, they would not have been able to feed their civilian population and armies, nor continued to make explosives. The First World War might have ended as a direct result of embargo of essential raw materials. With the aid of organic chemistry, however, the war continued. Nowadays, most nitrates are produced from ammonia synthesized from atmospheric nitrogen.
Chemical properties
The nitrate ion is a polyatomic ion with the empirical formula NO3− and a molecular mass of 62.0049. It consists of one central nitrogen atom surrounded by three identical oxygen atoms in a trigonal planar arrangement. The nitrate ion, which carries a formal charge of −1, can be represented as a "hybrid" of the following three resonance structures:
The structure may also be represented in the form of the diagram on the right.
The nitrate ion is the conjugate base of nitric acid. A nitrate salt forms when a positively charged ion (such as a metal ion) attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound. Almost all nitrates are soluble in water at standard temperature and pressure.
In organic chemistry, a nitrate is a functional group with the general chemical formula RONO2, where R stands for any organic residue. These nitrates are the esters of nitric acid and alcohols, formed by the process known as nitroxylation. Examples are:
- methyl nitrate, formed by reaction of methanol and nitric acid[1]
- the nitrate of tartaric acid[2]
- nitroglycerin.
Effects on aquatic life
In freshwater or estuarine systems close to land, nitrate concentrations can reach high levels, potentially causing the death of fish. Although the nitrate ion is much less toxic than ammonia or nitrite, levels over 30 parts per million (ppm) of nitrate can inhibit growth, impair the immune system, and cause stress in some aquatic species.
In most cases, high nitrate concentrations in aquatic systems are the result of surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. These levels of nitrate can also lead to algal blooms, and when nutrients (such as potassium, phosphate, or nitrate) become limiting, eutrophication can occur. Besides leading to water anoxia, these blooms may cause other changes to ecosystem functions, favoring some groups of organisms over others. Consequently, as nitrates form a component of total dissolved solids, they are widely used as indicators of water quality.
Specific nitrates
Ammonium nitrate
Ammonium nitrate (NH4NO3) is commonly used in agriculture as a high-nitrogen fertilizer. It can also be used as an oxidizing agent in explosives, especially improvised explosive devices.
Potassium nitrate
Potassium nitrate (KNO3) is a naturally occurring mineral source of nitrogen. Its common names include saltpeter (saltpetre), nitrate of potash, and nitre. It is used in the production of nitric acid, model rocket propellants, and several types of fireworks. In addition, it is a fertilizer and food preservative. Although also used in gunpowder, it is not combustible or flammable by itself.
Sodium nitrate
Sodium nitrate (NaNO3) is a type of salt that has long been used as an ingredient in explosives and solid rocket propellants, in glass and pottery enamel, and as a food preservative (such as in hot dogs), and has been mined extensively for these purposes. It is also variously known as caliche, Chile saltpeter, saltpeter, and soda niter. Chile has the largest reserves of caliche. It can also be manufactured synthetically.
Silver nitrate
Silver nitrate (AgNO3) is a soluble salt of silver and a corrosive compound. It produces a gray or black stain on skin. As a light-sensitive material, it is used in preparing photographic film. It is also used in making silver-based explosives and in staining biological samples for research. In addition, it has been used in medicine for its antiseptic properties.
Related materials
- Nitrates should not be confused with nitrites, the salts of nitrous acid.
- Organic compounds containing the nitro (NO2) functional group are known as nitro compounds.
See also
Notes
- ↑ Black, Alvin P., and Frank H. Babers. "Methyl Nitrate." Organic Syntheses 2 (1943): 412; 19 (1939): 64. Methyl Nitrate Retrieved September 13, 2007.
- ↑ Snyder, H.R., et al., "Imidazole." Organic Syntheses 3 (1955): 471; 22 (1942): 65. Imidazole Retrieved September 13, 2007.
ReferencesISBN links support NWE through referral fees
- Addiscott, T.M., A.J. Gold, C.A. Oviatt, N. Benjamin, and K.E. Giller. Nitrate, Agriculture and the Environment. CABI Publishing, 2005. ISBN 0851999131
- Chang, Raymond. Chemistry. 9th ed. New York: McGraw-Hill Science/Engineering/Math, 2006. ISBN 0073221031
- Cotton, F. Albert, and Geoffrey Wilkinson. Advanced Inorganic Chemistry. 4th ed. New York: Wiley, 1980. ISBN 0-471-02775-8
- Razowska-Jaworek, Lidia, and Andrzej Sadurski. Nitrates in Groundwater. International Association of Hydrogeologists Selected Papers. Leiden, the Netherlands: A.A. Balkema, 2005. ISBN 9058096645
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Nitrate history
- Ammonium_nitrate history
- Potassium_nitrate history
- Sodium_nitrate history
- Silver_nitrate history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.