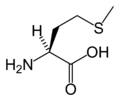
Methionine
| Methionine | |
|---|---|
| Systematic name | (S)-2-amino-4-(methylsulfanyl)- butanoic acid |
| Abbreviations | Met M |
| Chemical formula | C5H11NO2S |
| Molecular mass | 149.21 g mol-1 |
| Melting point | 281 °C |
| Density | 1.340 g cm-3 |
| Isoelectric point | 5.74 |
| pKa | 2.16 9.08 |
| CAS number | [63-68-3] |
| PubChem | 876 |
| EINECS number | 200-562-9 |
| SMILES | CSCC[C@H](N)C(O)=O |
| |
| Disclaimer and references | |
Methionine is an α-amino acid present in many proteins and, together with cysteine, is one of two sulfur-containing proteinogenic amino acids.
Its derivative S-adenosyl methionine (SAM) serves as a methyl donor. Methionine plays a role in the biosynthesis of cysteine, carnitine, and taurine (by the transsulfuration pathway), lecithin production, the synthesis of phosphatidylcholine, and other phospholipids. Improper conversion of methionine can lead to atherosclerosis.
The L-isomer of methionine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Methionine also is classified as an "essential amino acid" since it cannot be synthesized by the human body from other compounds through chemical reactions and thus has to be taken in with the diet.
Methionine's three letter code is Met, its one letter code is M, and its systematic name is 2-Amino-4-(methylthio)butanoic acid. Methionine is one of only two amino acids encoded by a single codon (AUG) in the standard genetic code (tryptophan, encoded by UGG, is the other). The codon AUG is also significant, in that it carries the "Start" message for a ribosome to begin protein translation from mRNA. As a consequence, methionine is incorporated into the N-terminal position of all proteins in eukaryotes and archaea during translation, although it is usually removed by post-translational modification.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In lysine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Lysine's chemical formula is NH2-(CH2)4- CH(NH2)-COOH, or in general form C6H14N2O2 (IUPAC-IUB 1983).
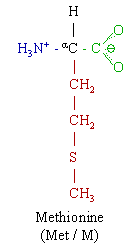
the chemical formula HO2CCH(NH2)CH2CH2SCH3.
acid CH3-S-[CH2]2-CH(NH2)-COOH
Methionine This essential is classified as nonpolar. Always the first amino acid to be incorporated into a protein; sometimes removed after translation. Like cysteine, it contains sulfur, but with a methyl group instead of hydrogen. This methyl group can be activated, and is used in many reactions where a new carbon atom is being added to another molecule.
Lysine is a basic amino acid, as are arginine and histidine. Lysine behaves similarly to arginine. It contains a long flexible side-chain with a positively-charged end. The flexibility of the chain makes lysine and arginine suitable for binding to molecules with many negative charges on their surfaces; for example, DNA-binding proteins have their active regions rich with arginine and lysine. The strong charge makes these two amino acids prone to be located on the outer hydrophilic surfaces of the proteins.
The ε-amino group often participates in hydrogen bonding and as a general base in catalysis. Common posttranslational modifications include methylation of the e-amino group, giving methyl-, dimethyl-, and trimethyllysine. The latter occurs in calmodulin. Other posttranslational modifications include acetylation. Collagen contains hydroxylysine, which is derived from lysine by lysyl hydroxylase. O-Glycosylation of lysine residues in the endoplasmic reticulum or Golgi apparatus is used to mark certain proteins for secretion from the cell.
Sources
As an essential amino acid, lysine is not synthesized in animals, hence it must be ingested as lysine or lysine-containing proteins. The human nutritional requirement is 1–1.5 g daily.
Biosynthesis
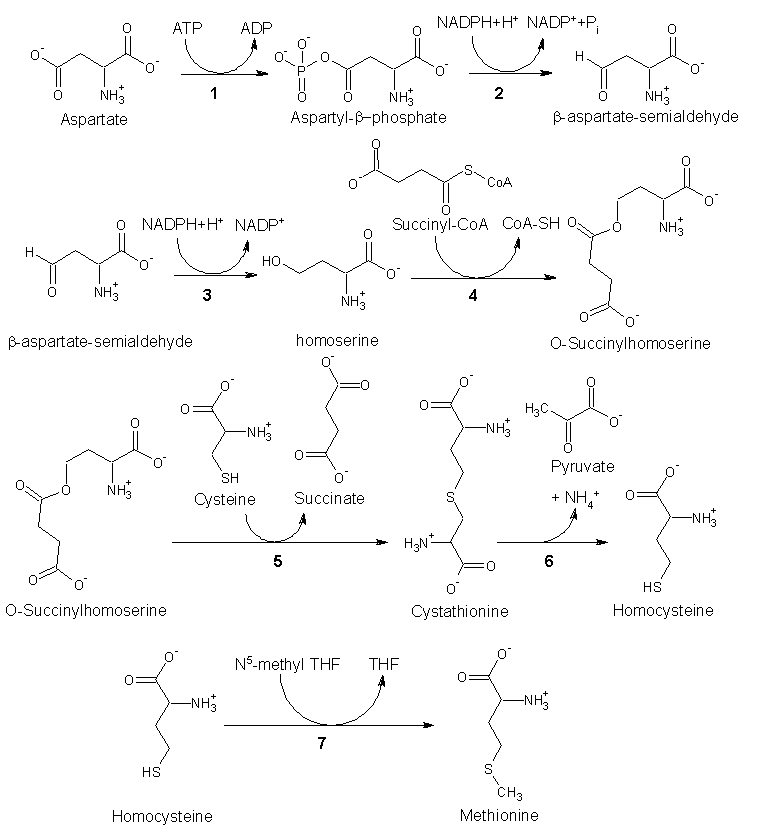
As an essential amino acid, methionine is not synthesized in humans, hence we must ingest methionine or methionine-containing proteins. In plants and microorganisms, methionine is synthesized via a pathway that uses both aspartic acid and cysteine. First, aspartic acid is converted via β-aspartyl-semialdehyde into homoserine, introducing the pair of contiguous methylene groups. Homoserine converts to O-succinyl homoserine, which then reacts with cysteine to produce cystathionine, which is cleaved to yield homocysteine. Subsequent methylation of the thiol group by folates affords methionine. Both cystathionine-γ-synthase and cystathionine-β-lyase require Pyridoxyl-5'-phosphate as a cofactor, whereas homocysteine methyltransferase requires Vitamin B12 as a cofactor.[1]
Enzymes involved in methionine biosynthesis:
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine acyltransferase
- cystathionine-γ-synthase
- cystathionine-β-lyase
- methionine synthase (in mammals, this step is performed by homocysteine methyltransferase)
Other biochemical pathways
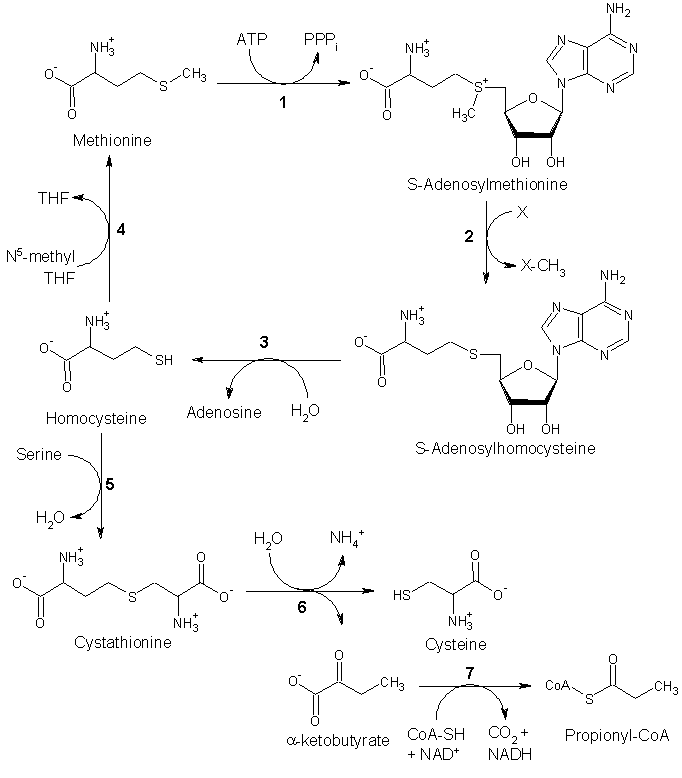
Although mammals cannot synthesize methionine, they can still utilize it in a variety of biochemical pathways:
Methionine is converted to S-adenosylmethionine (SAM) by (1) methionine adenosyltransferase. SAM serves as a methyl-donor in many (2) methyltransferase reactions and is converted to S-adenosylhomocysteine (SAH). (3) adenosylhomocysteinase converts SAH to homocysteine.
There are two fates of homocysteine.
- First, methionine can be regenerated from homocysteine via (4) methionine synthase. It can also be remethylated using glycine betaine (NNN-trimethyl glycine) to methionine via the enzyme Betaine-homocysteine methyltransferase (E.C.2.1.1.5, BHMT). BHMT makes up to 1.5% of all the soluble protein of the liver, and recent evidence suggests that it may have a greater influence on methionine and homocysteine homeostasis than Methionine sythase.
- Alternatively, homocysteine can be converted to cysteine. (5) cystathionine-β-synthase (a PLP-dependent enzyme) combines homocysteine and serine to produce cystathionine. Instead of degrading cystathionine via cystathionine-β-lyase as in the biosynthetic pathway, cystathionine is broken down to cysteine and α-ketobutyrate via (6) cystathionine-γ-lyase. (7) α-ketoacid dehydrogenase converts α-ketobutyrate to propionyl-CoA, which is metabolized to succinyl-CoA in a three-step process (see propionyl-CoA for pathway).
Synthesis
Racemic methionine can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2), by alkylation with chloroethylmethylsulfide, ClCH2CH2SCH3 followed by hydrolysis and decarboxylation.[2]
Dietary aspects
High levels of methionine can be found in sesame seeds, Brazil nuts, fish, meat, and some seeds. Most fruit and vegetables contain very little, although peppers and spinach are the best sources.
See also
- Allantoin
- Formylmethionine
ReferencesISBN links support NWE through referral fees
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Barger, G.; Weichselbaum, T. E. “dl-Methionine” Organic Syntheses, Collected Volume 2, p.384 (1943). http://www.orgsyn.org/orgsyn/pdfs/CV2P0384.pdf.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Key Supplements (KS). 2007 L-Arginine supplements nitric oxide scientific studies food sources. Key Supplements. Retrieved February 20, 2007.
- Lebret, T., J. M. Hervéa, P. Gornyb, M. Worcelc, and H. Botto. 2002. Efficacy and safety of a novel combination of L-arginine glutamate and yohimbine hydrochloride: A new oral therapy for erectile dysfunction. European Urology 41(6): 608-613.
- Longe, J. L. (Ed.) 2005. The Gale Encyclopedia of Alternative Medicine. Detroit: Thomson/Gale. ISBN 0787693960.
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.