Fructose
Fructose (or levulose) is a simple sugar (monosaccharide) with the chemical formula (C6H12O6); it is an isomer of glucose. Along with glucose and galactose, fructose is one of the three most important blood sugars in animals.
Sources of fructose include honey, fruits, and some root vegetables. Fructose is often found in combination with glucose as the disaccharide sucrose, a readily transportable and mobilizable sugar that is stored in many plant cells, such as in sugar beets and sugar cane. In animals, fructose may also be utilized as an energy source, and phosphate derivatives of fructose participate in the metabolism of carbohydrates.
Fructose is often recommended for, and consumed by, people with diabetes mellitus or hypoglycemia (low blood sugar), because it has a very low Glycemic Index (GI 23) relative to sucrose. However, this benefit is tempered by concern that fructose may have an adverse effect on plasma lipid and uric acid levels, and the resulting higher blood levels of fructose can be damaging to proteins.
The commercial use of fructose as a sweetener: High-fructose corn syrups Often Fructose is consumed as high fructose corn syrup which is corn syrup (glucose) which has been enzymatically treated, by the enzyme glucose isomerase, to convert a portion of the glucose into fructose thus making it sweeter. This is done to such a degree to yield corn syrup with an equivalent sweetness as sucrose by weight. While most carbohydrates have around the same amount of calories, fructose is sweeter, so manufacturers may use less fructose to get the same sweetness. concerns
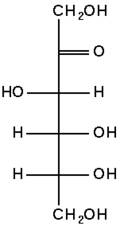
The chemical structure of fructose
Fructose is a levorotatory monosaccharide with the same empirical formula as glucose but with a different structure (i.e., it is an isomer of glucose). Like glucose, fructose is a hexose (six-carbon) sugar, but it contains a keto group instead of an aldehyde group, making it a ketohexose.
Like glucose, the fructose can also exist in ring form. Its open-chain structure is able to cyclize (form a ring) because a ketone can react with an alcohol to form a hemiketal. Specifically, the C-2 keto group in the open-chain form of fructose can react with its C-5 hydroxyl group to form an intramolecular hemiketal. Thus, although fructose is a hexose, it forms a five-membered ring called a furanose, a structure that predominates in solution.
Fructose's specific conformation (or structure) is responsible for its unique physical and chemical properties relative to glucose. Although the perception of sweetness depends on a variety of factors, such as concentration, pH, temperature, and individual taste buds, fructose is estimated to be approximately 1.2-1.8 times sweeter than glucose.
Fructose as an energy source
Much of our dietary fructose is dealt with in the liver, a control point for the circulation of blood sugar. Most compounds absorbed by the intestine pass through the liver, which enables it to regulate the level of many metabolites in the blood.
Two pathways for metabolizing fructose so that it enters the glycolytic pathway; different pathways preferred by the liver and adipose tissue. Can be used but requires a few additional modifications. Glycolysis is a series of biochemical reactions by which one molecule of glucose (Glc) is oxidized to two molecules of pyruvic acid (Pyr), yielding a small net gain of chemical energy to power cellular function. However, for aerobic organisms such as humans, glycolysis is only the initial stage of carbohydrate catabolism; the end-products of glycolysis enter into the citric acid cycle (also known as the TCA or Krebs cycle) and the electron transport chain for further oxidation. These pathways together produce considerably more energy per glucose molecule than anaerobic oxidation.
Much dietary fructose is metab’d by the liver, using the fructose-1-phosphate pathway. 1. Phosphorylation by fructokinase. Split into glyceraldehyde and dihydroxyacetone phosphate (an aldol cleavage). Glyceraldehyde is then photophorylated by triose kinase so that it too can enter glycolysis
Alt: fructose can be phosphorylated to fructose-6-phosphate by hexokinase. Little f6p is formed in the liver given the abundance of glucose rel to fructose in this organ. In contrast, adipose has much more fru than glu; most of the fru in adipose is thus mobilized by this route.
Disorders involving fructose metabolism
Fructose depends on glucose to carry it into the blood stream via GLUT-5 and then GLUT-2 [1]. Absorption of fructose without glucose present is very poor, and excess fructose is carried into the lower intestine where it provides nutrients for the existing flora, which produce gas. It may also cause water retention in the intestine. These effects may lead to bloating, excessive flatulence, loose stools, and even diarrhea depending on the amounts eaten and other factors.
Fructose has been hypothesized to cause obesity [2], elevated LDL cholesterol and triglycerides, leading to metabolic syndrome. Unlike animal experiments, some human experiments have failed to show a correlation between fructose consumption and obesity. Short term tests, lack of dietary control, and lack of a non-fructose consuming control group are all confounding factors in human experiments. However, there are now a number of reports showing correlation of fructose consumption to obesity, especially central obesity which is generally regarded as the most dangerous type. (Wylie-Rosett, 2004)(Havel, 2005)(Bray, 2004) (Dennison, 1997)
Fructose also chelates minerals in the blood. This effect is especially important with micronutrients such as copper, chromium and zinc. Since these solutes are normally present in small quantities, chelation of small numbers of ions may lead to deficiency diseases, immune system impairment and even insulin resistance, a component of type II diabetes (Higdon).
Fructose is a reducing sugar, as are all monosaccharides. The spontaneous addition of single sugar molecules to proteins, known as glycation, is a significant cause of damage in diabetics. Fructose appears to be as dangerous as glucose in this regard and so does not seem to be the answer for diabetes (McPherson et al, 1988) This may be an important contribution to senescence and many age-related chronic diseases (Levi & Werman 1998).
Fructose intolerance (Hereditary Fructose Intolerance, or HFI) is a hereditary condition due to a deficiency of liver enzymes that metabolise fructose. The deficient enzyme is Fructose-1-phosphate aldolase-B, this means that the fructose cannot be further metabolised beyond fructose-1-phosphate. This traps phosphates; which are needed to phosphorolyse glycogen phosphorolase to carry on to make glucose. Therefore glucose cannot be made through the breakdown of glycogen nor from gluconeogenesis, resulting in severe hypoglycaemia. If fructose is ingested, vomiting, hypoglycaemia and eventually kidney failure will follow.
Hereditary Fructose Intolerance should not be confused with fructose malabsorption or Dietary Fructose Intolerance (DFI), a deficiency of fructose transporter enzyme in the enterocytes, which leads to abdominal bloating, diarrhea and/or constipation.
Fructose malabsorption is a dietary disability of the small intestine in which the fructose carrier in enterocytes is deficient. Medical tests are similar as in lactose intolerance, requiring a hydrogen breath test for a clinical diagnosis.
Fructose Malabsorption is not to be confused with fructose intolerance or Hereditary Fructose Intolerance (HFI), a hereditary condition in which the liver enzymes that break fructose up are deficient. In patients with fructose malabsorption, the small intestine fails to absorb fructose properly. In the large intestine the unabsorbed fructose osmotically reduces the absorption of water and is metabolized by normal colonic bacteria to short chain fatty acids and the gases hydrogen, carbon dioxide and methane. The abnormal increase in hydrogen is detected with the hydrogen breath test.
There is no known cure, but an appropriate diet will help. However, it is very difficult for undiagnosed sufferers to see any relationship between the foods they eat and the symptoms they suffer, even if they keep a daily diet diary. This is because most foods contain a mixture of fructose and glucose. Foods with more fructose than glucose are a problem, as are foods with a lot of fructose (regardless of the amount of glucose). However, depending upon the sufferer's sensitivity to fructose, small amounts of problem foods could be eaten (especially when they are not the main ingredient of a meal).
Foods with a high glucose content actually help sufferers absorb fructose.
The commercial use of high fructose corn syrup
Production
High fructose corn syrup (HFCS) is a newer and sweeter form of corn syrup. Like ordinary corn syrup, the high fructose variety is made from corn starch using enzymes. The production process of HFCS was developed by Japanese researchers in the 1970s. HFCS was rapidly introduced in many processed foods and soda drinks in the US over the period of about 1975–1985, and usage continues to increase as sugar use decreases at a nearly one to one level (Bray, 2004 & U.S. Department of Agriculture, Economic Research Service, Sugar and Sweetener Yearbook series, Tables 50–52.). There are three main reasons for this switch; first is cost, as HFCS is a bit cheaper due to corn subsidies and import sugar tariffs. The second reason is that it is a liquid which is easier to blend and transport. The third is that a product made with HFCS has a much longer shelf life. (White JS. 1992. Fructose syrup: production, properties and applications, in FW Schenck & RE Hebeda, eds, Starch Hydrolysis Products – Worldwide Technology, Production, and Applications. VCH Publishers, Inc. 177-200)
By increasing the fructose content of corn syrup (glucose) through enzymatic processing, the syrup is more comparable to table sugar (sucrose). This makes it useful to manufacturers as a possible substitute for sugar in soft drinks and other processed foods. Common commercial grades of high fructose corn syrup include fructose contents of 42%, 55%, or 90%. The 55% grade is most commonly used in soft drinks and equivalent to caster sugar.
Unlike sucrose, HFCS consists of a mixture of glucose and fructose, which doesn't require an enzymatic step to break it down before absorption in the intestine.
There are currently suspicions that over-consumption of HFCS may be a main contributor to the epidemic of diabetes in the US. [3]
Comparison to other sugars
Sucrose (table sugar) is a disaccharide composed of one unit each of fructose and glucose linked together. Sucrose is 50% fructose, so HFCS may have a higher or lower fructose content than sucrose, with a corresponding change in sweetness. Sucrose is broken down during digestion into fructose and glucose through hydrolysis by the enzyme sucrase.
Honey is another product that is a mixture of different types of sugars, water, and small amounts of other compounds. Honey typically has a fructose/glucose ratio similar to HFCS, as well as containing some sucrose and other sugars.
Sweetener consumption patterns
The accompanying graph shows the consumption of sweeteners per capita in the United States since 1966. Since HFCS and sucrose (cane and beet sugars) provide almost identical proportions of fructose and glucose, no metabolic changes would be expected from substituting one for the other. However, it is apparent from this graph that overall sweetener consumption, and in particular glucose-fructose mixtures, has increased since the introduction of HFCS. Thus, the proportion of fructose as a component of overall sweetener intake in the United States has increased since the early 1980s. This would be true whether the added sweetener was HFCS, table sugar, or any other glucose-fructose mixture.
HFCS is produced in the industrialized countries.The production of HFCS is dependent on the agricultural, especially sugar, policy.
In Europe, due to the fact that HFCS (isoglucose) is under the adjustment of production, the greater availability of cane sugar over maize would make its production there uneconomical.
In Japan, HFCS consumption accounts for one quarter of total sweetener consumption.
The potential impact on human health
The average American consumed approximately 19.2 kg of HFCS versus 20 kg of sugar in 2004.[citation needed] Where HFCS is not used or rarely used, the sugar consumption per person can be higher than the USA; for example, the 2002 figures for some countries are: USA 32.4 kg, EU 40.1 kg, Brazil 59.7 kg, and Australia 56.2 kg.[4]
One study concluded that fructose "produced significantly higher fasting plasma triacylglycerol values than did the glucose diet in men" and "if plasma triacylglycerols are a risk factor for cardiovascular disease, then diets high in fructose may be undesirable"[5]. A study in mice suggests that fructose increases adiposity.[6] However, these studies looked at the effects of fructose alone. As noted by the U.S. Food and Drug Administration in 1996, the saccharide composition (glucose to fructose ratio) of HFCS is approximately the same as that of honey, invert sugar and the disaccharide sucrose (or table sugar).
A more recent study found a link exists between obesity and high HFCS consumption, especially from soft drinks.[7]
However, the obesity epidemic has many contributing factors. University of California, Davis nutrition researcher Peter Havel has pointed out that while there are likely differences between sweeteners, "the increased consumption of fat, the increased consumption of all sugars, and inactivity are all to blame for the obesity epidemic."[8]
ReferencesISBN links support NWE through referral fees
- ↑ Buchs, AE and Sasson S, Joost HG, Cerasi E. (1998). Characterization of GLUT5 domains responsible for fructose transport. Endocrinology 139: 827-31. PMID 12399260.
- ↑ Elliott, B and Keim NL, Stern JS, Teff K, Havel PJ (2002). Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 76: 911-22. PMID 12399260.
- ↑ Sugar coated: We're drowning in high fructose corn syrup.
- ↑ WHO Oral Health Country/Area Profile Programme Global Sugar Consumption
- ↑ Bantle, John P. and Susan K. Raatz, William Thomas and Angeliki Georgopoulos (November 2000). Effects of dietary fructose on plasma lipids in healthy subjects. American Journal of Clinical Nutrition 72 (5): 1128-1134.
- ↑ Jurgens, Hella and et al. (2005). Consuming Fructose-sweetened Beverages Increases Body Adiposity in Mice. Obesity Res 13: 1146-1156.
- ↑ Bray, George A. and Samara Joy Nielsen and Barry M. Popkin (April 2004). Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. American Journal of Clinical Nutrition 79 (4): 537-543.
- ↑ Warner, Melanie, "A Sweetener With a Bad Rap", New York Times, 2006-07-02. Retrieved 2006-10-03.
- Levi B, Werman MJ. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J Nutr 1998;128:1442-9. Fulltext. PMID 9732303.
- McPherson JD, Shilton BH, Walton DJ. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988;27:1901-7. PMID 3132203.
- Higdon, J., Linus Pauling Institute, Oregon State U. Chromium 2003
- Wylie-Rosett,Judith, et al, Carbohydrates and Increases in Obesity: Does the Type of Carbohydrate Make a Difference? Obesity Research 12:124S-129S (2004)[1]
- Havel, PJ, Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005 May;63(5):133-57 [2]
- Bray, George A, Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity American Journal of Clinical Nutrition, Vol. 79, No. 4, 537-543, April 2004 [3]
- Dennison, Barbara Excess Fruit Juice Consumption by Preschool-aged Children Is Associated With Short Stature and Obesity, PEDIATRICS Vol. 99 No.1, January 1997, pp. 15-22 [4]
External links
Template:ChemicalSources
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.