Difference between revisions of "Cytosine" - New World Encyclopedia
Katya Swarts (talk | contribs) |
m ({{Contracted}}) |
||
| Line 1: | Line 1: | ||
| + | {{Contracted}} | ||
<!-- Here is a table of datad; skip past it to edit the text. —> | <!-- Here is a table of datad; skip past it to edit the text. —> | ||
<!-- <nowiki> Submit {{subst:chembox_simple_organic}} to get this template or go to [[Template:Chembox_simple_organic]]. </nowiki> —> | <!-- <nowiki> Submit {{subst:chembox_simple_organic}} to get this template or go to [[Template:Chembox_simple_organic]]. </nowiki> —> | ||
Revision as of 15:08, 26 December 2006
| Cytosine | |
|---|---|
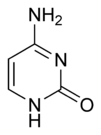
| Chemical name | 4-Aminopyrimidin-2(1H)-one |
| Chemical formula | C4H5N3O |
| Molecular mass | 111.102 g/mol |
| Melting point | 320 - 325°C (decomp) |
| CAS number | 71-30-7 |
| SMILES | NC1=NC(NC=C1)=O |
| |
Cytosine is one of the 5 main nucleobases used in storing and transporting genetic information within a cell in the nucleic acids DNA and RNA. It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an amine group at position 4 and a keto group at position 2). The nucleoside of cytosine is cytidine. In Watson-Crick base pairing, it forms three hydrogen bonds with guanine.
Cytosine was first discovered in 1894 when it was isolated from calf thymus tissues. A structure was proposed in 1903, and was synthesized (and thus confirmed) in the laboratory in the same year.
Cytosine recently found use in quantum computation. The first time any quantum mechanical properties were harnessed to process information took place on August 1st in 1998 when researchers at Oxford implemented David Deutsch's algorithm on a two qubit NMRQC (Nuclear Magnetic Resonance Quantum Computer) based on the cytosine molecule.
Cytosine can be found as part of DNA, RNA, or as a part of a nucleotide. As cytidine triphosphate (CTP), it can act as a co-factor to enzymes, and can transfer a phosphate to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP).
In DNA and RNA, cytosine is paired with guanine. However, it is inherently unstable, and can change into uracil (spontaneous deamination). This can lead to a point mutation if not repaired by the DNA repair enzymes such as uracil glycosylase, which cleaves a uracil in DNA.
Cytosine can also be methylated into 5-methylcytosine by an enzyme called DNA methyltransferase.
External links
- CID 597 from PubChem — 4-amino-3H-pyrimidin-2-one
- CID 5274263 from PubChem — 4-aminopyrimidin-2-ol
- EINECS number 200-749-5
- Computational Chemistry Wiki
- Prebiotic cytosine synthesis: A critical analysis and implications for the origin of life
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - mtDNA - Oligonucleotide |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.