Difference between revisions of "Crystal" - New World Encyclopedia
m |
(added credit and category tags, deleted foreign language links) |
||
| Line 1: | Line 1: | ||
| − | + | {{dablink|For other senses of this word, see [[crystal (disambiguation)]].}} | |
| + | [[image:Quartz Crystal.jpg|thumb|250px|[[Quartz]] crystal]] | ||
| − | [[ | + | In [[chemistry]] and [[mineralogy]], a '''crystal''' is a [[solid]] in which the constituent [[atom]]s, [[molecule]]s, or [[ion]]s are packed in a regularly ordered, repeating pattern extending in all three spatial dimensions. |
| − | + | Generally, crystals form when they undergo a process of solidification. Under ideal conditions, the result may be a single crystal, where all of the atoms in the solid fit into the same crystal structure. However, generally, many crystals form simultaneously during solidification, leading to a polycrystalline solid. For example, most metals encountered in everyday life are polycrystals. Crystals are often symmetrically intergrown to form crystal twins. | |
| − | + | Which [[crystal structure]] the fluid will form depends on the [[chemistry]] of the fluid, the conditions under which it is being solidified, and also on the ambient [[pressure]]. The process of forming a crystalline structure is often referred to as '''[[crystallization]]'''. | |
| − | + | [[image:Bismuth_crystal_macro.jpg|thumb|250px|Synthetic [[bismuth]] crystal]] | |
| − | [[ | + | While the cooling process usually results in the generation of a crystalline material, under certain conditions, the fluid may be frozen in a noncrystalline state. In most cases, this involves cooling the fluid so rapidly that atoms cannot travel to their lattice sites before they lose mobility. A noncrystalline material, which has no [[long-range order]], is called an [[amorphous]], [[vitreous]], or [[glass]]y material. It is also often referred to as an amorphous solid, although there are distinct differences between solids and glasses: most notably, the process of forming a glass does not release the [[latent heat of fusion]]. For this reason, many scientists consider glassy materials to be [[viscosity|viscous]] liquids rather than solids, although this is a controversial topic; see the entry on [[glass]] for more details. |
| − | + | [[Image:Insulincrystals.jpg|thumb|250px|[[Insulin]] crystals]] | |
| − | |||
| − | [[Image:Insulincrystals.jpg|thumb| | ||
Crystalline structures occur in all classes of materials, with all types of [[chemical bond]]s. Almost all [[metallic bond | metal]] exists in a polycrystalline state; amorphous or single-crystal metals must be produced synthetically, often with great difficulty. [[ionic bond | Ionically bonded]] crystals can form upon solidification of salts, either from a molten fluid or when it condenses from a solution. [[Covalent]]ly bonded crystals are also very common, notable examples being diamond, silica, and graphite. [[Polymer]] materials generally will form crystalline regions, but the lengths of the molecules usually prevents complete crystallization. Weak [[Van der Waals force]]s can also play a role in a crystal structure; for example, this type of bonding loosely holds together the hexagonal-patterned sheets in [[graphite]]. | Crystalline structures occur in all classes of materials, with all types of [[chemical bond]]s. Almost all [[metallic bond | metal]] exists in a polycrystalline state; amorphous or single-crystal metals must be produced synthetically, often with great difficulty. [[ionic bond | Ionically bonded]] crystals can form upon solidification of salts, either from a molten fluid or when it condenses from a solution. [[Covalent]]ly bonded crystals are also very common, notable examples being diamond, silica, and graphite. [[Polymer]] materials generally will form crystalline regions, but the lengths of the molecules usually prevents complete crystallization. Weak [[Van der Waals force]]s can also play a role in a crystal structure; for example, this type of bonding loosely holds together the hexagonal-patterned sheets in [[graphite]]. | ||
| Line 19: | Line 18: | ||
Most crystalline materials have a variety of [[crystallographic defect]]s. The types and structures of these defects can have a profound effect on the properties of the materials. | Most crystalline materials have a variety of [[crystallographic defect]]s. The types and structures of these defects can have a profound effect on the properties of the materials. | ||
| − | [[Image:Gallium1_640x480.jpg|thumb| | + | [[Image:Gallium1_640x480.jpg|thumb|250px|[[Gallium]], a metal that easily forms large single crystals]] |
| − | [[Image:Monocristal dsc03676.jpg|thumb| | + | [[Image:Monocristal dsc03676.jpg|thumb|250px|A huge monocrystal of [[potassium dihydrogen phosphate]] grown from solution by [[Saint-Gobain]] for the [[megajoule laser]] of [[Commissariat à l'Énergie Atomique|CEA]].]] |
| − | While the term "crystal" has a precise meaning within [[materials science]] and [[solid-state physics]], colloquially "crystal" refers to solid objects that exhibit well-defined and often pleasing geometric shapes. | + | While the term "crystal" has a precise meaning within [[materials science]] and [[solid-state physics]], colloquially "crystal" refers to solid objects that exhibit well-defined and often pleasing geometric shapes. In this sense of the word, many types of crystals are found in nature. The shape of these crystals is dependent on the types of molecular bonds between the atoms to determine the structure, as well as on the conditions under which they formed. [[Snowflake]]s, [[diamond]]s, and common [[salt]] are common examples of crystals. |
| − | Some crystalline materials may exhibit special electrical properties such as the [[ferroelectric effect]] or the [[ | + | Some crystalline materials may exhibit special electrical properties such as the [[ferroelectric effect]] or the [[piezoelectricity|piezoelectric effect]]. Additionally, [[light]] passing through a crystal is often bent in different directions, producing an array of [[color]]s; [[crystal optics]] is the study of these effects. In periodic [[dielectric]] structures a range of unique optical properties can be expected as described in [[photonic crystal]]s. |
| − | + | [[Crystallography]] is the scientific study of crystals and crystal formation. | |
| + | ==Historical and mythical uses== | ||
| + | According to [[Bahya_ben_Asher|Rebbenu Bachya]], the word "Achlmah" in the verse [[Exodus]] 28:19 means "Crystal" and was the stone on the [[Ephod]] representing the tribe of [[Gad]]. | ||
| − | + | Crystals also figure or figured prominently as healing tools in a number of mythologies. | |
== See also == | == See also == | ||
| + | |||
| + | * [[Atomic packing factor]] | ||
* [[Crystal habit]] | * [[Crystal habit]] | ||
* [[Crystal structure]] | * [[Crystal structure]] | ||
* [[Crystallite]] | * [[Crystallite]] | ||
| + | * [[Crystallization]] | ||
* [[Liquid crystal]] | * [[Liquid crystal]] | ||
* [[Quasicrystal]] | * [[Quasicrystal]] | ||
* [[Seed crystal]] | * [[Seed crystal]] | ||
* [[Single crystal]] | * [[Single crystal]] | ||
| + | * [[Polymorphism (materials science)]] | ||
| + | * [[Crystal ball]] | ||
| + | * [[Crystal oscillator]] | ||
| + | * [[Crystal radio]] | ||
==External links== | ==External links== | ||
| − | + | * [http://chemistry.tidalswan.com/index.php?title=Crystal_and_molecular_architecture Chemistry of Crystals] | |
* [http://www.rockhounds.com/rockshop/xtal/index.html Introduction to Crystallography and Mineral Crystal Systems] | * [http://www.rockhounds.com/rockshop/xtal/index.html Introduction to Crystallography and Mineral Crystal Systems] | ||
* [http://www.iucr.ac.uk/iucr-top/comm/cteach/pamphlets.html Crystallographic Teaching Pamphlets] | * [http://www.iucr.ac.uk/iucr-top/comm/cteach/pamphlets.html Crystallographic Teaching Pamphlets] | ||
* [http://cst-www.nrl.navy.mil/lattice/spcgrp/ Crystal Lattice Structures] | * [http://cst-www.nrl.navy.mil/lattice/spcgrp/ Crystal Lattice Structures] | ||
| + | * [http://www.thecrystalweb.org/ A virtual museum about the crystal] | ||
| + | * [http://giantcrystals.strahlen.org The Giant Crystal Project - documenting the largest crystals and crystal aggregates known to exist] | ||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
| − | [[Category: | + | [[Category:Earth sciences]] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | {{credit| | + | {{credit|79916637}} |
Revision as of 22:31, 6 October 2006
- For other senses of this word, see crystal (disambiguation).

In chemistry and mineralogy, a crystal is a solid in which the constituent atoms, molecules, or ions are packed in a regularly ordered, repeating pattern extending in all three spatial dimensions.
Generally, crystals form when they undergo a process of solidification. Under ideal conditions, the result may be a single crystal, where all of the atoms in the solid fit into the same crystal structure. However, generally, many crystals form simultaneously during solidification, leading to a polycrystalline solid. For example, most metals encountered in everyday life are polycrystals. Crystals are often symmetrically intergrown to form crystal twins.
Which crystal structure the fluid will form depends on the chemistry of the fluid, the conditions under which it is being solidified, and also on the ambient pressure. The process of forming a crystalline structure is often referred to as crystallization.

While the cooling process usually results in the generation of a crystalline material, under certain conditions, the fluid may be frozen in a noncrystalline state. In most cases, this involves cooling the fluid so rapidly that atoms cannot travel to their lattice sites before they lose mobility. A noncrystalline material, which has no long-range order, is called an amorphous, vitreous, or glassy material. It is also often referred to as an amorphous solid, although there are distinct differences between solids and glasses: most notably, the process of forming a glass does not release the latent heat of fusion. For this reason, many scientists consider glassy materials to be viscous liquids rather than solids, although this is a controversial topic; see the entry on glass for more details.
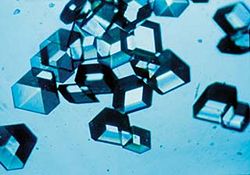
Crystalline structures occur in all classes of materials, with all types of chemical bonds. Almost all metal exists in a polycrystalline state; amorphous or single-crystal metals must be produced synthetically, often with great difficulty. Ionically bonded crystals can form upon solidification of salts, either from a molten fluid or when it condenses from a solution. Covalently bonded crystals are also very common, notable examples being diamond, silica, and graphite. Polymer materials generally will form crystalline regions, but the lengths of the molecules usually prevents complete crystallization. Weak Van der Waals forces can also play a role in a crystal structure; for example, this type of bonding loosely holds together the hexagonal-patterned sheets in graphite.
Most crystalline materials have a variety of crystallographic defects. The types and structures of these defects can have a profound effect on the properties of the materials.

While the term "crystal" has a precise meaning within materials science and solid-state physics, colloquially "crystal" refers to solid objects that exhibit well-defined and often pleasing geometric shapes. In this sense of the word, many types of crystals are found in nature. The shape of these crystals is dependent on the types of molecular bonds between the atoms to determine the structure, as well as on the conditions under which they formed. Snowflakes, diamonds, and common salt are common examples of crystals.
Some crystalline materials may exhibit special electrical properties such as the ferroelectric effect or the piezoelectric effect. Additionally, light passing through a crystal is often bent in different directions, producing an array of colors; crystal optics is the study of these effects. In periodic dielectric structures a range of unique optical properties can be expected as described in photonic crystals.
Crystallography is the scientific study of crystals and crystal formation.
Historical and mythical uses
According to Rebbenu Bachya, the word "Achlmah" in the verse Exodus 28:19 means "Crystal" and was the stone on the Ephod representing the tribe of Gad.
Crystals also figure or figured prominently as healing tools in a number of mythologies.
See also
- Atomic packing factor
- Crystal habit
- Crystal structure
- Crystallite
- Crystallization
- Liquid crystal
- Quasicrystal
- Seed crystal
- Single crystal
- Polymorphism (materials science)
- Crystal ball
- Crystal oscillator
- Crystal radio
External links
- Chemistry of Crystals
- Introduction to Crystallography and Mineral Crystal Systems
- Crystallographic Teaching Pamphlets
- Crystal Lattice Structures
- A virtual museum about the crystal
- The Giant Crystal Project - documenting the largest crystals and crystal aggregates known to exist
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
