Adenine
| Adenine | |
|---|---|
| Chemical name | 9H-Purin-6-amine |
| Alternate name | 6-aminopurine |
| Chemical formula | C5H5N5 |
| Molecular mass | 135.13 g/mol |
| Melting point | 360 - 365 °C |
| CAS number | 73-24-5 |
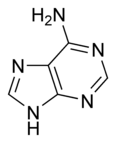
| SMILES | NC1=NC=NC2=C1N=CN2 |
| |
Adenine is one of the two purine nucleobases used in forming nucleotides of the nucleic acids DNA and RNA. In DNA, adenine (A) binds to thymine (T) via two hydrogen bonds to assist in stabilizing the nucleic acid structures. In RNA, adenine binds to uracil (U).
Adenine forms adenosine, a nucleoside, when attached to ribose, and deoxyadenosine when attached to deoxyribose; it forms adenosine triphosphate (ATP), a nucleotide, when three phosphate groups are added to adenosine. Adenosine triphosphate is used in cellular metabolism as one of the basic methods of transferring chemical energy between reactions.
In older literature, adenine was sometimes called Vitamin B4. However it is no longer considered a true vitamin (see Vitamin B).
Some think that, at the origin of life on Earth, the first adenine was formed by the polymerizing of five hydrogen cyanide (HCN) molecules.
Adenine is one of the two purine nucleobases used in forming nucleotides of the nucleic acids DNA and RNA. In DNA , adenine (A) binds to thymine (T) via two hydrogen bonds to assist in stabilizing the nucleic acid structures. In RNA , adenine binds to uracil (U). Adenine and thymine, together with cytosine and guanine, the two pyrimidine nucleobases, are the four “letters” that code for cellular synthesis of amino acids, the buildng blocks of proteins.
In the human body adenine is synthesized in the liver. The vitamin folic acid is essential for adenine synthesis.
Adenine forms adenosine , a nucleoside, when attached to ribose , and deoxyadenosine when attached to deoxyribose ; it forms adenosine triphosphate (ATP), a nucleotide , when three phosphate groups are added to adenosine .Adenosine triphosphate is used in cellular metabolism as one of the basic methods of transferring chemical energy between reactions.
In older literature, adenine was sometimes called Vitamin B 4. However it is no longer considered a true vitamin (see Vitamin B ).
Some think that, at the origin of life on Earth, the first adenine was formed by the polymerizing of five hydrogen cyanide (HCN) molecules.
External link
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - mtDNA - Oligonucleotide |
| Vitamins |
|---|
| All B vitamins | All D vitamins |
| Retinol (A) | Thiamine (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic acid (B5) | Pyridoxine (B6) | Biotin (B7) | Folic acid (B9) | Cyanocobalamin (B12) | Ascorbic acid (C) | Ergocalciferol (D2) | Cholecalciferol (D3) | Tocopherol (E) | Naphthoquinone (K) |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.