Ribose
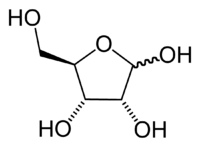
Ribose, primarily seen as D-ribose, is a water-soluable, pentose sugar (monosaccharide with five carbon atoms) that is an important component of nucleic acids, nucleotides, the vitamin riboflavin, and various co-enzymes. Ribose has the chemical formula C5H10O5.
This ubiquitous sugar and its derivatives are fundamental to key biological processes throughout nature and reflect a commonality among all living organisms.
Ribonucleic acid (RNA) is a nucleic acid based on the sugar ribose. Deoxyribonucleic acid (DNA) is a nucleic acid based on the closely related sugar deoxyribose. The bases in these nucleic acids (adenine, uracil, guanine, and cytosine in RNA, and thymine instead of uracil in DNA) represents the genetic information in living cells. As a component of RNA, which is used for genetic transcription, ribose is critical to living creatures.
Ribose is also a component of the nucleotide ATP, the coenzyme NADH, and several other chemicals that are critical to metabolism.
Structure
Ribose is an aldopentose, which means a pentose sugar with an aldehyde functional group in position one. An aldehyde group consists of a carbon atom that is bonded to a hydrogen atom and double-bonded to an oxygen atom (chemical formula O=CH-).
Ribose forms a five-member ring composed of four carbon atoms and one oxygen. Hydroxyl (-OH) groups are attached to three of the carbons. The fourth carbon in the ring (one of the carbon atoms adjacent to the oxygen) has attached to it the fifth carbon atom and a hydroxyl group.
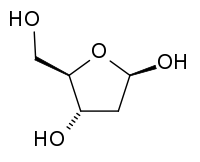
Deoxyribose, also known as 2-deoxyribose, is also an aldopentose. It is derived from ribose by the replacement of the hydroxyl group at the two position (the carbon furthest from the attached carbon) with hydrogen, leading to the net loss of an oxygen atom. Deoxyribose has the chemical formula C5H10O4.
Ribose was discovered in 1909 by Phoebus Levene, who also discovered DNA (1929) and found that DNA contained adenine, guanine, thymine, cytosine, deoxyribose, and a phosphate group.
Biological importance of ribose
Ribose and derivatives have an important role in biology.
Among the most important derivatives are those with phosphate groups attached at the five position. Mono-, di-, and triphosphate forms are important, as well as 3-5 cyclic monophosphates.
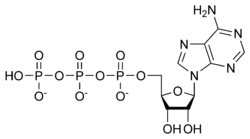
There are important diphosphate dimers called coenzymes that purines and pyrimidines form with ribose. When these purine and pyrimidine derivatives are coupled to a ribose sugar, they are called nucleosides. In these compounds, the convention is to put a ′ (pronounced "prime") after the carbon numbers of the sugar, so that in nucleoside derivatives a name might include, for instance, the term "5′-monophosphate," meaning that the phosphate group is attached to the fifth carbon of the sugar, and not to the base. The bases are attached to the 1′ribose carbon in the common nucleosides.
Phosphorylated nucleosides are called nucleotides.
The most common bases in nucleotides are:
- The purines adenine and guanine;
- The pyrimidines cytosine, thymine, and uracil; and
- The pyridine nicotinamide.
The sugar component is either ribose or deoxyribose. (‚ÄúDeoxy‚ÄĚ simply indicates that the sugar lacks an oxygen atom present in ribose, the parent compound.) Depending on their base sugar, nucleotides are therefore known as ‚Äúdeoxyribonucleotides‚ÄĚ or ‚Äúribonucleotides.‚ÄĚ The nucleic acid DNA is built of nucleotides with a deoxyribose sugar, whereas RNA contains nucleotides composed of ribose sugars.
One of the common bases is adenine (a purine derivative); coupled to ribose it is called adenosine. The 5′-triphosphate derivative of adenosine is commonly called ATP, for adenosine triphosphate. As the name suggests, the structure of this nucleotide consists of a purine base (adenine), a ribose sugar, and three phosphate groups. While ATP is one of four nucleotides required for the synthesis of ribonucleic acids, it is primarily known in biochemistry for its role in metabolism as the "molecular currency" of intracellular energy transfer.
Ribose nucleotides are often found in unbranched 5′-3′ polymers. In these structures, the 3′carbon of one monomer unit is linked to a phosphate that is attached to the 5′carbon of the next unit, and so on. These polymer chains often contain many millions of monomer units. Since long polymers have physical properties distinctly different from those of small molecules, they are called macromolecules. The sugar-phosphate-sugar chain is called the backbone of the polymer. One end of the backbone has a free 5′phosphate, and the other end has a free 3′OH group. The backbone structure is independent of which particular bases are attached to the individual sugars.
Genetic material often contains poly 5′-3′, 2′-deoxyribose nucleotides, in structures called chromosomes, where each monomer is one of the nucleotides deoxy- adenine, thymine, guanine, or cytosine. This material is called deoxyribonucleic acid, or simply DNA for short. DNA in chromosomes forms very long helical structures containing two molecules with the backbones running in opposite directions on the outside of the helix and held together by hydrogen bonds between complementary nucleotide bases lying between the helical backbones.
In contrast, very similar molecules, containing ribose instead of deoxyribose, and known generically as RNA, are known to form only relatively short double-helical complementary base paired structures, utilizing uracil rather than thymine. These are well known, for instance, in ribosomal RNA molecules and in transfer RNA (tRNA), where so-called hairpin structures form from palindromic sequences within one molecule.
Riboflavin (vitamin B2) also utilizes ribose in its synthesis. Riboflavin in key to maintaining good health and is required in many cellular processes, including energy metabolism and metabolism of carbohydrates, proteins, and fats.
ReferencesISBN links support NWE through referral fees
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1989. Molecular Biology of the Cell. New York: Garland Publishing. ISBN 0824036956
- Doonan, S. 2004. Nucleic Acids. Great Britain: Royal Society of Chemistry. ISBN 0854044817
- Stryer, L. 1995. Biochemistry, 4th edition. New York, NY: W.H. Freeman.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.