Difference between revisions of "Isoleucine" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 13: | Line 13: | ||
'''Isoleucine''' is an α-[[amino acid]] with the [[chemical formula]] HO<sub>2</sub>CCH(NH<sub>2</sub>)CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>3</sub>. Its three letter code is ILE and its one letter code is I. It is an [[Essential amino acid|essential]] amino acid, which means that humans cannot synthesise it, so it must be part of our diet. With a hydrocarbon side chain, Isoleucine is classified as a [[hydrophobic]] amino acid. | '''Isoleucine''' is an α-[[amino acid]] with the [[chemical formula]] HO<sub>2</sub>CCH(NH<sub>2</sub>)CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>3</sub>. Its three letter code is ILE and its one letter code is I. It is an [[Essential amino acid|essential]] amino acid, which means that humans cannot synthesise it, so it must be part of our diet. With a hydrocarbon side chain, Isoleucine is classified as a [[hydrophobic]] amino acid. | ||
| − | Together with [[threonine]], isoleucine is one of two common amino acids that has a [[Chirality (chemistry)|chiral]] side chain. Four [[stereoisomer]]s of isoleucine are possible, including two possible [[diastereomer]]s of <small>L</small>-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2''S'',3''S'')-2-amino-3-methylpentanoic acid. | + | Together with [[threonine]], isoleucine is one of two common amino acids that has a [[Chirality (chemistry)|chiral]] side chain; that is, one that is not superimposable on its mirror image. Four [[stereoisomer]]s of isoleucine are possible, including two possible [[diastereomer]]s of <small>L</small>-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2''S'',3''S'')-2-amino-3-methylpentanoic acid. |
| + | |||
| + | '''Valine''' is an α-[[amino acid]] that is found in most proteins and is essential in the human diet. It is similar to [[leucine]] and [[isoleucine]] in being a branched-chain amino acid and whose buildup in the blood and urine, due a particular [[enzyme]] deficiency, causes the serious metabolic disorder [[#maple syrup urine disease|maple syrup urine disease]]. | ||
| + | |||
| + | In humans, the L-isomer of valine, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] common in animal proteins and required for normal functioning in humans. Valine is also classified as an [[amino acid#essential amino acid|"essential amino acid"]] since it cannot be synthesized by the [[human body]] from other compounds through chemical reactions and thus has to be taken in with the diet. | ||
| + | |||
| + | The precision and complex coordination in the universe is revealed in valine's role in proteins. Similar to [[leucine]] and [[isoleucine]], valine's structure makes it important for the correct folding of [[protein]]s. The functionality of a protein is dependent upon its ability to fold into a precise three-dimensional shape. In [[sickle-cell disease]], valine substitutes for the [[hydrophilic]] (binds with water) amino acid [[glutamic acid]] in [[hemoglobin]]. Because valine is [[hydrophobic]] (repelled by water), the hemoglobin does not fold correctly. | ||
| + | |||
| + | In the case of essential amino acids, it is important for individuals to have disciplined eating habits in order to get proper amounts. This is emphasized in the case of maple syrup urine disorder, where one must obtain minimal levels of valine (and leucine and isoleucine) without consuming too much to lead to the symptoms. | ||
| + | |||
| + | Valine's three letter code is Val, its one letter code is V, its [[codon]]s are GUU, GUC, GUA, and GUG, and its systematic name is 2-Amino-3-methylbutanoic acid (IUPAC-IUB 1983). Valine is named after the plant [[Valerian (herb)|valerian]]. | ||
| + | |||
| + | ==Structure== | ||
| + | In [[biochemistry]], the term [[amino acid]] is frequently used to refer specifically to ''alpha amino acids'': those amino acids in which the amino and carboxylate groups are attached to the same [[carbon]], the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is: | ||
| + | |||
| + | ''R'' | ||
| + | | | ||
| + | H<sub>2</sub>N-C-COOH | ||
| + | | | ||
| + | H | ||
| + | where ''R'' represents a ''side chain'' specific to each amino acid. | ||
| + | |||
| + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In valine, only the L-stereoisomer is involved in synthesis of [[mammal]]ian proteins. | ||
| + | |||
| + | Valine's chemical formula is (CH<sub>3</sub>)<sub>2</sub>CH-CH(NH<sub>2</sub>)-COOH, or in general form C<sub>5</sub>H<sub>11</sub>NO<sub>2</sub> (IUPAC-IUB 1983). | ||
| + | |||
| + | Like [[isoleucine]] and [[leucine]], valine has large aliphatic hydrophobic side chains. Its molecules are rigid, and its mutual hydrophobic interactions are important for the correct folding of proteins, as these chains tend to be located inside of the protein molecule. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
== Biosynthesis == | == Biosynthesis == | ||
| Line 52: | Line 83: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319. | ||
| + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | ||
| + | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry'', 3rd ed. New York: Worth Publishing. ISBN 1572591536. | ||
==External links== | ==External links== | ||
Revision as of 17:49, 17 June 2007
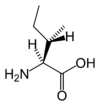  Chemical structure of L-isoleucine | |
Isoleucine | |
| Systematic (IUPAC) name | |
| (2S,3S)-2-amino-3-methylpentanoic acid | |
| Identifiers | |
| CAS number | 73-32-5 |
| PubChem | 791 |
| Chemical data | |
| Formula | C6H13NO2 |
| Mol. weight | 131.18 |
| SMILES | CC[C@H](C)[C@H](N)C(O)=O |
| Complete data | |
Isoleucine is an α-amino acid with the chemical formula HO2CCH(NH2)CH(CH3)CH2CH3. Its three letter code is ILE and its one letter code is I. It is an essential amino acid, which means that humans cannot synthesise it, so it must be part of our diet. With a hydrocarbon side chain, Isoleucine is classified as a hydrophobic amino acid.
Together with threonine, isoleucine is one of two common amino acids that has a chiral side chain; that is, one that is not superimposable on its mirror image. Four stereoisomers of isoleucine are possible, including two possible diastereomers of L-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2S,3S)-2-amino-3-methylpentanoic acid.
Valine is an α-amino acid that is found in most proteins and is essential in the human diet. It is similar to leucine and isoleucine in being a branched-chain amino acid and whose buildup in the blood and urine, due a particular enzyme deficiency, causes the serious metabolic disorder maple syrup urine disease.
In humans, the L-isomer of valine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Valine is also classified as an "essential amino acid" since it cannot be synthesized by the human body from other compounds through chemical reactions and thus has to be taken in with the diet.
The precision and complex coordination in the universe is revealed in valine's role in proteins. Similar to leucine and isoleucine, valine's structure makes it important for the correct folding of proteins. The functionality of a protein is dependent upon its ability to fold into a precise three-dimensional shape. In sickle-cell disease, valine substitutes for the hydrophilic (binds with water) amino acid glutamic acid in hemoglobin. Because valine is hydrophobic (repelled by water), the hemoglobin does not fold correctly.
In the case of essential amino acids, it is important for individuals to have disciplined eating habits in order to get proper amounts. This is emphasized in the case of maple syrup urine disorder, where one must obtain minimal levels of valine (and leucine and isoleucine) without consuming too much to lead to the symptoms.
Valine's three letter code is Val, its one letter code is V, its codons are GUU, GUC, GUA, and GUG, and its systematic name is 2-Amino-3-methylbutanoic acid (IUPAC-IUB 1983). Valine is named after the plant valerian.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In valine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Valine's chemical formula is (CH3)2CH-CH(NH2)-COOH, or in general form C5H11NO2 (IUPAC-IUB 1983).
Like isoleucine and leucine, valine has large aliphatic hydrophobic side chains. Its molecules are rigid, and its mutual hydrophobic interactions are important for the correct folding of proteins, as these chains tend to be located inside of the protein molecule.
Biosynthesis
As an essential amino acid, isoleucine is not synthesized in animals, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps starting from pyruvic acid and alpha-ketoglutarate. Enzymes involved in this biosynthesis include:[1]
- acetolactate synthase
- acetohydroxy acid isomeroreductase
- dihydroxyacid dehydratase
- valine aminotransferase
Isomers of isoleucine
| Forms of Isoleucine | |||||||
|---|---|---|---|---|---|---|---|
| Common name: | isoleucine | D-isoleucine | L-isoleucine | DL-isoleucine | allo-D-isoleucine | allo-L-isoleucine | allo-DL-isoleucine |
| Synonyms: | (R)-Isoleucine | L(+)-Isoleucine | (R*,R*)-isoleucine | alloisoleucine | |||
| PubChem: | CID 791 | CID 94206 | CID 6306 | CID 76551 | |||
| EINECS number: | 207-139-8 | 206-269-2 | 200-798-2 | 216-143-9 | 216-142-3 | 221-464-2
| |
| CAS number: | 443-79-8 | 319-78-8 | 73-32-5 | 1509-35-9 | 1509-34-8 | 3107-04-8 | |
Synthesis
Isoleucine can be synthesized in a multistep procedure starting from 2-bromobutane and diethylmalonate.[2] Synthetic isoleucine was originally reported in 1905.[3]
Dietary aspects
Rich sources of isoleucine are eggs, chicken, pork, mutton, pulses, soya beans, cottage cheese, milk, piyal seeds, cashew nuts, and cereal grains.
ReferencesISBN links support NWE through referral fees
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Marvel, C. S. “dl-Isoleucine” Organic Syntheses, Collected Volume 3, p.495 (1955). http://www.orgsyn.org/orgsyn/pdfs/CV3P0495.pdf
- ↑ Bouveault and Locquin, Compt. rend., 141, 115 (1905).
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.