High-intensity discharge lamp
High-intensity discharge (HID) lamps include several types of electrical lamps: mercury-vapor, metal halide (also HQI), high-pressure sodium, low-pressure sodium and less common, xenon short-arc lamps. The light-producing element of these lamp types is a well-stabilized arc discharge contained within a refractory envelope (arc tube) with wall loading in excess of 3 watts-per-square-centimeter (W/cm²) (19.4 watts per square inch (W/in.²)).
Compared with fluorescent and incandescent lamps, HID lamps produce a far higher quantity of light per unit area of lamp package.
Construction
HID lamps produce light by striking an electrical arc across tungsten electrodes housed inside a specially designed inner fused quartz or fused alumina tube. This tube is filled with both gas and metals. The gas aids in the starting of the lamps. Then, the metals produce the light once they are heated to a point of evaporation, forming a plasma.
Types of HID lamps include:
- Mercury vapor (color rendering index (CRI) range 15-55)
- Metal halide (CRI range 65-80, ceramic MH can go to 90s)
- Low-pressure sodium (CRI 0 owing to their monochromatic light)
- High-pressure sodium (CRI range 22-75)
- Xenon arc lamps.
Mercury vapor lamps, which originally produced a bluish-green light, were the first commercially available HID lamps. Today, they are also available in a color corrected, whiter light. But they are still often being replaced by the newer, more efficient high-pressure sodium and metal halide lamps. Standard low-pressure sodium lamps have the highest efficiency of all HID lamps, but they produce a yellowish light. High-pressure sodium lamps that produce a whiter light are now available, but efficiency is somewhat sacrificed. Metal halide lamps are less efficient but produce an even whiter, more natural light. Colored metal halide lamps are also available.
Auxiliary devices
Like fluorescent lamps, HID lamps require a ballast to start and maintain their arcs. The method used to initially strike the arc varies: mercury vapor lamps and some metal halide lamps are usually started using a third electrode near one of the main electrodes while other lamp styles are usually started using pulses of high voltage.
Applications
HID lamps are typically used when high levels of light over large areas are required, and when energy efficiency and/or light intensity are desired. These areas include gymnasiums, large public areas, warehouses, movie theaters, outdoor activity areas, roadways, parking lots, and pathways. More recently, HID lamps, especially metal halide, have been used in small retail and residential environments. HID lamps have made indoor gardening practical, especially for plants that require a good deal of high intensity sunlight, like vegetables and flowers. They are also used to reproduce tropical intensity sunlight for indoor aquaria.
Some HID lamps such as Mercury Vapor Discharge produce large amounts of UV radiation and therefore need diffusers to block that radiation. In the last few years there have been several cases of faulty diffusers, causing people to suffer severe sunburn and Arc eye. Regulations may now require guarded lamps or lamps which will quickly burn out if their outer envelope is broken.
Recently, HID lamps have gained use in motor-vehicle headlamps. This application has met with mixed responses from motorists, mainly in response to the amount of glare that HID lights can cause. They often have an automatic self-leveling system to minimise this issue and as such are usually an expensive optional extra on most cars. However, many motorists still prefer these lights as they emit a clearer, brighter, more natural appearing light than normal headlamps.
HID lamps are used in high-end bicycle headlamps. They are desirable because they produce much more light than a halogen lamp of the same wattage. Halogen lights appear somewhat yellow in color; HID bicycle lights look faintly blue-violet.
HID lamps are also being used on many general aviation aircraft for landing and taxi lights.
Mercury-vapor lamp
A mercury-vapor lamp is a gas discharge lamp that uses mercury in an excited state to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger borosilicate glass bulb. The outer bulb may be clear or coated with a phosphor; in either case, the outer bulb provides thermal insulation, protection from ultraviolet radiation, and a convenient mounting for the fused quartz arc tube.
Mercury vapor lamps (and their relatives) are often used because they are relatively efficient. Phosphor coated bulbs offer better color rendition than either high- or low-pressure sodium vapor lamps. They also offer a very long lifetime, as well as intense lighting for several applications.
Theory and relations
The mercury-vapor lamp is a negative resistance device and requires auxiliary components (for example, a ballast) to prevent it from taking excessive current. The auxiliary components are substantially similar to the ballasts used with fluorescent lamps. It is used often for outside lighting (signs) and for auditoriums and stages.
Also like fluorescent lamps, mercury-vapor lamps usually require a starter, which is usually contained within the mercury vapor lamp itself. A third electrode is mounted near one of the main electrodes and connected through a resistor to the other main electrode. When power is applied, there is sufficient voltage to strike an arc between the starting electrode and the adjacent main electrode. This arc discharge eventually provides enough ionized mercury to strike an arc between the main electrodes. Occasionally, a thermal switch will also be installed to short the starting electrode to the adjacent main electrode, completely suppressing the starting arc once the main arc strikes.
Operation
When the lamp is first turned on, mercury-vapor lamps will produce a dark blue glow because only a small amount of the mercury is ionized and the gas pressure in the arc tube is very low (so much of the light is produced in the ultraviolet mercury bands). As the main arc strikes and the gas heats up and increases in pressure, the light shifts into the visible range and the high gas pressure causes the mercury emission bands to broaden somewhat, producing a light that appears more-white to the human eye (although it is still not a continuous spectrum). Even at full intensity, the light from a mercury vapor lamp with no phosphors is distinctly bluish in color.
Color considerations
To correct the bluish tinge, many mercury-vapor lamps are coated on the inside of the outer bulb with a phosphor that converts some portion of the ultraviolet emissions into red light. This helps fill in the otherwise very-deficient red end of the electromagnetic spectrum. These lamps are generally called "color corrected" lamps. Most modern mercury-vapor lamps have this coating. One of the original complaints against mercury lights was they tended to make people look like "bloodless corpses" because of the lack of light from the red end of the spectrum. There is also an increase in red color (e.g., due to the continuous radiation) in ultra-high pressure mercury vapor lamps (usually greater than 200 atm.) which has found application in modern compact projection devices.
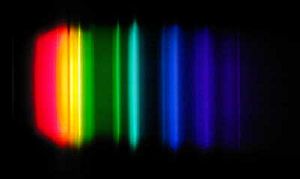
Emits Wavelengths - 253.7, 365.4, 404.7, 435.8, 546.1, and 578.0 nm.
Ultraviolet hazards
All mercury vapor lamps (including metal halide lamps) must contain a feature (or be installed in a fixture that contains a feature) that prevents ultraviolet radiation from escaping. Usually, the borosilicate glass outer bulb of the lamp performs this function but special care must be taken if the lamp is installed in a situation where this outer envelope can become damaged. There have been documented cases of lamps being damaged in gymnasiums and sun burns and eye inflammation have resulted.[1] When used in locations like gyms, the fixture should contain a strong outer guard or an outer lens to protect the lamp's outer bulb. Also, special "safety" lamps are made which will deliberately burn out if the outer glass is broken. This is usually achieved by a thin carbon strip used to connect one of the electrodes, which will burn up in the presence of air.
Even with these methods, some UV radiation can still pass through the outer bulb of the lamp. This causes the aging process of some plastics used in the construction of luminaires to be sped up, leaving them horribly discolored after only a few years' service. Polycarbonate suffers particularly from this problem; and it is not uncommon to see fairly new polycarbonate surfaces positioned near the lamp to have turned a dull, 'ear-wax'-like color after only a short time. Certain polishes, such as Brasso, can be used to remove some of the yellowing, but usually only with a limited success.
Metal halide lamp
Metal halide lamps, a member of the high-intensity discharge (HID) family of lamps, produce high light output for their size, making them a compact, powerful, and efficient light source. Originally created in the late 1960s for industrial use, metal halide lamps are now available in numerous sizes and configurations for commercial and residential applications. Like most HID lamps, metal halide lamps operate under high pressure and temperature, and require special fixtures to operate safely. They are also considered a "point" light source, so reflective luminaires are often required to concentrate the light for purposes of the lighting application.
Uses
Metal-halide lamps are used both for general industrial purposes, and for very specific applications which require specific UV or blue-frequency light. They are used for indoor growing applications, because they can provide the spectrum and temperature of light which encourage general plant growth. They are most often used in athletic facilities.

Operation
Like other gas-discharge lamps such as the very-similar mercury-vapor lamps, metal halide lamps produce light by passing an electric arc through a mixture of gases. In a metal halide lamp, the compact arc tube contains a high-pressure mixture of argon, mercury, and a variety of metal halides. The mixture of halides will affect the nature of light produced, influencing the correlated color temperature and intensity (making the light bluer, or redder, for example). The argon gas in the lamp is easily ionized, and facilitates striking the arc across the two electrodes when voltage is first applied to the lamp. The heat generated by the arc then vaporizes the mercury and metal halides, which produce light as the temperature and pressure increases.
Like all other gas discharge lamps, metal halide lamps require auxiliary equipment to provide proper starting and operating voltages and regulate the current flow in the lamp.
About 24 percent of the energy used by metal halide lamps produces light (65-115 lm/W[2]), making them generally more efficient than fluorescent lamps, and substantially more efficient than incandescent bulbs.
Components
Metal halide lamps consist of the following main components. They have a metal base (in some cases they are double-ended) that allows an electrical connection. They are covered with an outer glass shield (or glass bulb) to protect the inner components and provide a shield to UV light generated by the mercury vapor. Inside the glass shield, a series of support and lead wires hold the inner fused quartz arc tube and its embedded tungsten electrodes. It is within the arc tube that the light is actually created. Besides the mercury-vapor, the lamp contains iodides or sometimes bromides of different metals and noble gas. The composition of the metals used defines the color of the lamp.
Many types have alumina arc tube instead of quartz like high pressure sodium lamps have. They are usually referred as ceramic metal halide or CMH.
Some bulbs have a phosphor coating on the inner side of the outer bulb to diffuse the light.
Ballasts
Metal halide lamps require electrical ballasts to regulate the arc current flow and deliver the proper voltage to the arc. Probe start metal halide bulbs contain a special 'starting' electrode within the lamp to initiate the arc when the lamp is first lit (which generates a slight flicker when the lamp is first turned on). Pulse start metal halide lamps do not require a starting electrode, and instead use a special starting circuit referred to as an ignitor to generate a high-voltage pulse to the operating electrodes. American National Standards Institute (ANSI) lamp-ballast system standards establish parameters for all metal halide components (with the exception of some newer products).
A few electronic ballasts are now available for metal halide lamps. The benefit of these ballasts is more precise management of the lamp's wattage, which provides more consistent color and longer lamp life. In some cases, electronic ballasts are reported to increase efficiency (i.e. reduce electrical usage). However with few exceptions, high-frequency operation does not increase lamp efficiency as in the case of high-output (HO) or very high-output (VHO) fluorescent bulbs. High frequency electronic operation does however allow for specially designed dimming metal halide ballast systems.
Color temperature
Metal halide lamps were initially preferred to mercury vapor lamps in instances where natural light was desired because of the whiter light generated (mercury vapor lamps generating light that was much bluer). However the distinction today is not as great. Some metal halide lamps can deliver very clean "white" light that has a color-rendering index (CRI) in the 1980s. With the introduction of specialized metal halide mixtures, metal halide lamps are now available that can have a correlated color temperature as low as 3,000K (very yellow) to 20,000K (very blue). Some specialized lamps have been created specifically for the spectral absorption needs of plants (hydroponics and indoor gardening) or animals (indoor aquariums). Perhaps the most important point to keep in mind is that, due to tolerances in the manufacturing process, color temperature can vary slightly from lamp to lamp, and the color properties of metal halide bulbs cannot be predicted with 100 percent accuracy. Moreover, per ANSI standards the color specifications of metal halide bulbs are measured after the bulb has been burned for 100 hours (seasoned). The color characteristics of a metal halide lamp will not conform to specifications until the bulb has been properly seasoned. Color temperature variance is seen greatest in "probe start" technology lamps (+/- 300 Kelvin). Newer metal halide technology, referred to as "pulse start," has improved color rendering and a more controlled kelvin variance (+/- 100-200 Kelvin). The color temperature of a metal halide lamp can also be affected by the electrical characteristics of the electrical system powering the bulb and manufacturing variances in the bulb itself. In a manner similar to an incandescent bulb, if a metal halide bulb is underpowered it will have a lower physical temperature and hence its light output will be warmer (more red). The inverse is true for an overpowered bulb. Moreover, the color properties of metal halide lamps often change over the lifetime of the bulb.
Starting and warm up
A cold metal halide lamp cannot immediately begin producing its full light capacity because the temperature and pressure in the inner arc chamber require time to reach full operating levels. Starting the initial argon arc sometimes takes a few seconds, and the warm up period can be as long as five minutes (depending upon lamp type). During this time the lamp exhibits different colors as the various metal halides vaporize in the arc chamber.
If power is interrupted, even briefly, the lamp's arc will extinguish, and the high pressure that exists in the hot arc tube will prevent re-striking the arc; a cool-down period of 5-10 minutes will be required before the lamp can be re-started. This is a major concern in some lighting applications where prolonged lighting interruption could create manufacturing shut-down or a safety issue. A few metal halide lamps are made with "instant restrike" capabilities that use a ballast with very high operating voltages (30,000 volts) to restart a hot lamp.
Sodium vapor lamp
A sodium vapor lamp is a gas discharge lamp that uses sodium in an excited state to produce light. There are two varieties of such lamps: low pressure and high pressure.
Low pressure sodium (LPS or SOX)
LPS Lamps (Low Pressure Sodium), also known as SOX Lamps (Sodium OXide), consist of an outer vacuum envelope of glass coated with an infrared reflecting layer of indium tin oxide, a semiconductor material that allows the visible light wavelengths out and keeps the infrared (heat) back. It has an inner borosilicate 2 ply glass U shaped tube containing sodium metal and a small amount of neon and argon gas Penning mixture to start the gas discharge, so when the lamp is turned on it emits a dim red/pink light to warm the sodium metal and within a few minutes it turns into the common bright orange/yellow color as the sodium metal vaporizes. These lamps produce a virtually monochromatic light in the 590 nm wavelength. As a result, objects have no color rendering under a LPS light and are seen only by their reflection of the 590 nm light (orange).
LPS lamps are the most efficient electrically powered light source when measured for photopic lighting conditions—up to 200 lm/W.[3]. As a result they are widely used for outdoor lighting such as street lights and security lighting where color rendition is viewed by many to be less important. LPS lamps are available with power ratings from 10 W up to 180 W, however length increases greatly with wattage creating problems for designers.
LPS lamps are more closely related to fluorescent lamps than to high-intensity discharge lamps, because they have a low–pressure, low–intensity discharge source and a linear lamp shape. Also, like fluorecents, they do not exhibit a bright arc as do other HID lamps. Rather, they emit a softer, luminous glow, resulting in less glare.
Another unique property of LPS lamps is that, unlike other lamp types, they do not decline in lumen output with age. As an example, Mercury Vapor HID lamps become very dull towards the end of their lives, to the point of being ineffective, whilst still drawing their full rated load of electricity. LPS lamps, however, do increase energy usage towards their end of life, which is usually rated around 18,000 hours for modern lamps.
High pressure sodium (HPS, SON)
High pressure sodium (HPS) lamps are smaller and contain some other elements (such as mercury), producing a dark pink glow when first struck, and a pinkish orange light when warmed up. (Some bulbs also briefly produce a pure to bluish white light in between. This is probably from the mercury glowing before the sodium is completely warmed up). The sodium D-line is the main source of light from the HPS lamp, and it is extremely pressure broadened by the high sodium pressures in the lamp, hence colors of objects under them can be distinguished. This leads them to be used in areas where good color rendering is important, or desired.
High pressure sodium lamps are quite efficient — about 100 lm/W, up to 150 lm/W, when measured for Photopic lighting conditions. They have been widely used for outdoor lighting such as streetlights and security lighting. Understanding the change in human color vision sensitivity from Photopic to Mesopic and Scotopic is essential for proper planning when designing lighting for roads.
Because of the extremely high chemical activity of the high pressure sodium arc, the arc tube is typically made of translucent aluminum oxide (alumina). This construction led General Electric to use the tradename "Lucalox" for their line of high-pressure sodium lamps.
White SON
A variation of the high pressure sodium, the White SON, introduced in 1986, has a higher pressure than the typical HPS lamp, producing a color temperature of around 2,700K, with a CRI of 85; greatly resembling the color of incandescent light.[4] These are often indoors in cafes and restaurants to create a certain atmosphere. However, these lamps come at the cost of higher purchase cost, shorter life, and lower light efficiency.
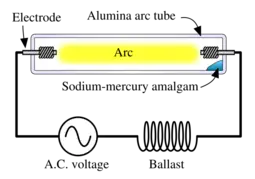
Theory of operation
An amalgam of metallic sodium and mercury lies at the coolest part of the lamp and provides the sodium and mercury vapor in which the arc is drawn. For a given voltage, there are generally three modes of operation:
- the lamp is extinguished and no current flows
- the lamp is operating with liquid amalgam in the tube
- the lamp is operating with all amalgam in the vapor state
The first and last states are stable, but the second state is unstable. Actual lamps are not designed to handle the power of the third state, this would result in catastrophic failure. Similarly, an anomalous drop in current will drive the lamp to extinction. It is the second state which is the desired operating state of the lamp. The result is an average lamp life in excess of 20,000 hours.
In practical use, the lamp is powered by an AC voltage source in series with an inductive "ballast" in order to supply a nearly constant current to the lamp, rather than a constant voltage, thus assuring stable operation. The ballast is usually inductive rather than simply being resistive which minimizes resistive losses. Also, since the lamp effectively extinguishes at each zero-current point in the AC cycle, the inductive ballast assists in the reignition by providing a voltage spike at the zero-current point.
LPS lamp failure does not result in cycling, rather, the lamp will simply not strike, and will maintain its dull red glow exhibited during the start up phase.
Xenon arc lamps
Xenon arc lamps use ionized xenon gas to produce a bright white light that closely mimics natural daylight. They can be roughly divided into three categories:
- Continuous-output xenon short-arc lamps
- Continuous-output xenon long-arc lamps
- Xenon flash lamps (which are usually considered separately)
Each consists of a glass or fused quartz arc tube with tungsten metal electrodes at each end. The glass tube is first evacuated and then re-filled with xenon gas. For xenon flashtubes, a third "trigger" electrode usually surrounds the exterior of the arc tube.
History and modern usage
Xenon short-arc lamps were invented in the 1940s in Germany and introduced in 1951 by Osram. First launched in the 2-kilowatt (kW) size (XBO2001), these lamps saw a wide acceptance in movie projection where it advantageously replaced the older carbon arc lamps. The white, continuous light generated with this arc is of daylight quality but plagued by a rather low lumen efficiency. Today, almost all movie projectors in theaters employ these lamps with a rating ranging from 900 W up to 12 kW. When used in Omnimax projection systems, the power can be as high as 15 kW in a single lamp.
Lamp construction
All modern xenon short-arc lamps utilize a fused quartz envelope with thorium-doped tungsten electrodes. Fused quartz is the only economically feasible material currently available that can withstand the high pressure and high temperature present in an operating lamp while still being optically clear. Because tungsten and quartz have different coefficients of thermal expansion, the tungsten electrodes are welded to strips of pure molybdenum metal or Invar alloy, which are then melted into the quartz to form the envelope seal.
Because of the very high power levels involved, the lamps may be water-cooled. In (continuous wave pumped) lasers the lamp is inserted into a fixed lamp jacket and the water flows between the jacket and the lamp. An O-ring seals off the tube, so that the naked electrodes do not get into contact with the water. In low power applications the electrodes are too cold for efficient electron emission and are not cooled, in high power applications an additional water cooling circuit for each electrode is necessary. To save costs, the water circuits are often not separated and the water needs to be highly deionized, which in turn lets the quartz or some laser mediums dissolve into the water.
In order to achieve maximum efficiency, the xenon gas inside a short-arc lamp has to be maintained at an extremely high pressure. With large lamps this presents a serious safety concern, because if the lamp is dropped or ruptures in service, pieces of the lamp envelope can be ejected at high velocity, causing bodily injury or death. To mitigate this risk, large xenon short-arc lamps are shipped inside special protective shields (see photograph), which will contain the envelope fragments if the lamp is dropped and explodes. When the lamp reaches the end of its useful life, the protective shield is put back on the lamp, and the spent lamp is then removed from the equipment and disposed of. The risk of explosion increases as the lamp is used.
There is another type of lamp known as a ceramic Xenon lamp (Developed by Perkin-Elmer as Cermax). It uses a ceramic lamp body with an integrated reflector.
Light generation mechanism
Xenon short-arc lamps come in two distinct varieties: pure xenon, which contain only xenon gas; and xenon-mercury, which contain xenon gas and a small amount of mercury metal.
In a pure xenon lamp, the majority of the light is generated within a tiny, pinpoint-sized cloud of plasma situated where the electron stream leaves the face of the cathode. The light generation volume is cone-shaped, and the luminous intensity falls off exponentially moving from cathode to anode. Electrons that manage to pass through the plasma cloud collide with the anode, causing it to heat up. As a result, the anode in a xenon short-arc lamp either has to be much larger than the cathode or be water-cooled, to safely dissipate the heat. Pure xenon short-arc lamps have a "near daylight" spectrum.
Even in a high pressure lamp, there are some very strong emission lines in the near infrared.
In xenon-mercury short-arc lamps, the majority of the light is generated within a tiny, pinpoint sized cloud of plasma situated at the tip of each electrode. The light generation volume is shaped like two intersecting cones, and the luminous intensity falls off exponentially moving towards the centre of the lamp. Xenon-mercury short-arc lamps have a bluish-white spectrum and extremely high UV output. These lamps are used primarily for UV curing applications, sterilizing objects, and generating ozone.
The very small optical size of the arc makes it possible to focus the light from the lamp very precisely. For this reason, xenon arc lamps of smaller sizes, down to 10 watts, are used in optics and in precision illumination for microscopes and other instruments. Larger lamps are also employed in searchlights where narrow beams of light are to be generated, or in film production lighting where daylight simulation is required.
All xenon short-arc lamps generate significant amounts of ultraviolet radiation while in operation. Xenon has strong spectral lines in the UV bands, and these readily pass through the fused quartz lamp envelope. Unlike the borosilicate glass used in standard lamps, fused quartz does not attenuate UV radiation. The UV radiation released by a short-arc lamp can cause a secondary problem of ozone generation. The UV radiation strikes oxygen molecules in the air surrounding the lamp, causing them to ionize. Some of the ionized molecules then recombine as O3, ozone. Equipment that uses short-arc lamps as the light source must be designed to contain UV radiation and prevent ozone build-up.
Many lamps have a low-UV blocking coating on the envelope and are sold as "Ozone Free" lamps. Some lamps have envelopes made out of ultra-pure synthetic fused silica (trade name "Suprasil"), which roughly doubles the cost, but which allows them to emit useful light into the so-called vacuum UV region. These lamps are normally operated in a pure Nitrogen atmosphere.
Power supply requirements
Xenon short-arc lamps are low-voltage, high-amperage, direct-current devices with a negative temperature coefficient. They require a high voltage pulse in the 50 kV range to start the lamp, and require extremely well regulated DC as the power source. They are also inherently unstable, prone to phenomena such as plasma oscillation and thermal runaway. Because of these characteristics, xenon short-arc lamps require a sophisticated power supply to achieve stable, long-life operation. The usual approach is to regulate the current flowing in the lamp rather than the applied voltage.
Applications
The use of the xenon technology has spread into the consumer market with the introduction in 1991 of xenon headlamps for cars. In this lamp, the glass capsule is small and the arc spans only a few millimeters. Additions of mercury and salts of sodium and scandium improve significantly the lumen output of the lamp, the xenon gas being used only to provide instant light upon the ignition of the lamp.
Xenon long-arc lamps
These are structurally similar to short-arc lamps except that the arc-containing portion of the glass tube is greatly elongated. When mounted within an elliptical reflector, these lamps are frequently used to simulate sunlight. Typical uses include solar cell testing, solar simulation for age testing of materials, rapid thermal processing, and material inspection.
Light pollution considerations
For placements where light pollution is of prime importance (for example, an observatory parking lot), low pressure sodium is preferred. As it emits light on only one wavelength, it is the easiest to filter out. Mercury-vapor lamps without any phosphor are second best; they produce only a few distinct mercury lines that need to be filtered out.
End of life
At the end of life, many types of high-intensity discharge lamps exhibit a phenomenon known as cycling. These lamps can be started at a relatively low voltage but as they heat up during operation, the internal gas pressure within the arc tube rises and more and more voltage is required to maintain the arc discharge. As a lamp gets older, the maintaining voltage for the arc eventually rises to exceed the voltage provided by the electrical ballast. As the lamp heats to this point, the arc fails and the lamp goes out. Eventually, with the arc extinguished, the lamp cools down again, the gas pressure in the arc tube is reduced, and the ballast can once again cause the arc to strike. The effect of this is that the lamp glows for a while and then goes out, repeatedly.
More-sophisticated ballast designs detect cycling and give up attempting to start the lamp after a few cycles. If power is removed and reapplied, the ballast will make a new series of startup attempts.
See also
Notes
- ↑ Thun, MJ and Altman R, Ellingson O, Mills LF, Talansky ML (Nov 1982). Ocular complications of malfunctioning mercury vapor lamps. Ann Ophthalmol. 14 (11): 1017-20. PMID: 7181332.
- ↑ http://www.venturelighting.com/TechCenter/Metal-Halide-TechIntro.html Retrieved April 24, 2007.
- ↑ http://webexhibits.org/causesofcolor/4.html Retrieved April 24, 2007.
- ↑ Philips SDW-T High Pressure Sodium White SON. J.D. Hooker. Retrieved May 5, 2008.
ReferencesISBN links support NWE through referral fees
- Waymouth, John (1971). Electric Discharge Lamps. Cambridge, MA: The M.I.T. Press. ISBN 0262230488.
- National Highway Traffic Safety Administration. Glare from headlamps and other front mounted lamps. Federal Motor Vehicle Safety Standard No. 108. US Department of Transportation. Retrieved January 23, 2006.
- de Groot, J.J. and J.A.J.M. van Vliet (1986). The High-Pressure Sodium Lamp. Antwerp: Kluwer Technische Bocken B.V.. ISBN 9020119028.
- Flesch, P. 2006. Light and Light Sources: High-Intensity Discharge Lamps. New York, NY: Springer. ISBN 3540326847
External links
All links retrieved December 24, 2017.
| Sources of light / lighting: | ||
|---|---|---|
|
Natural/prehistoric light sources: |
Bioluminescence | Celestial objects | Lightning |
|
|
Combustion-based light sources: |
Acetylene/Carbide lamps | Candles | Davy lamps | Fire | Gas lighting | Kerosene lamps | Lanterns | Limelights | Oil lamps | Rushlights | |
|
Nuclear/direct chemical light sources: |
Betalights/Trasers | Chemoluminescence (Lightsticks) | |
|
Electric light sources: |
Arc lamps | Incandescent light bulbs | Fluorescent lamps | |
|
High-intensity discharge light sources: |
Ceramic Discharge Metal Halide lamps | HMI lamps | Mercury-vapor lamps | Metal halide lamps | Sodium vapor lamps | Xenon arc lamps | |
|
Other electric light sources: |
Electroluminescent (EL) lamps | Globar | Inductive lighting | Discrete LEDs/Solid State Lighting (LEDs) | Neon and argon lamps | Nernst lamp | Sulfur lamp | Xenon flash lamps | Yablochkov candles | |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- High-intensity_discharge_lamp history
- Mercury-vapor_lamp history
- Metal_halide_lamp history
- Sodium_vapor_lamp history
- Xenon_arc_lamp history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.