Difference between revisions of "Photochemistry" - New World Encyclopedia
(added credit and category tags, deleted foreign language links) |
Rosie Tanabe (talk | contribs) |
||
| (11 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[Image:Spectre.svg|thumb|350px| | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{copyedited}} |
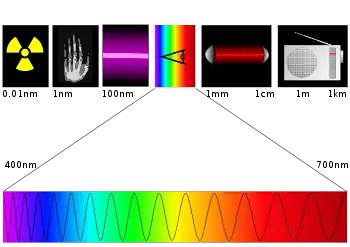
| + | [[Image:Spectre.svg|thumb|350px|Significant regions of the electromagnetic spectrum, with their approximate wavelengths. The bottom part of the illustration shows an expanded version of the visible spectrum.]] | ||
| − | + | '''Photochemistry,''' a sub-discipline of [[chemistry]], is the study of the interactions between [[atom]]s, [[molecule]]s, and [[light]] (or [[electromagnetic radiation]]).<ref>{{GoldBookRef|title=photochemistry|file=P04588}} Retrieved August 21, 2007.</ref> The chemical reactions that take place through these interactions are known as '''photochemical reactions.''' Examples of photochemical reactions are [[photosynthesis]] in [[plant]] cells and light-induced changes that take place in the [[eye]]. In addition, photochemical reactions are important in [[photography]], [[dye bleaching]], and [[television display]]s. | |
| + | {{toc}} | ||
| + | == Reactions activated by light == | ||
| + | |||
| + | A photochemical reaction may be thought of as a reaction ignited by the absorption of light. Normally, a reaction (not just a photochemical reaction) occurs when the molecules involved gain the [[activation energy]] necessary to undergo change. For example, for the [[combustion]] of [[gasoline]] (a [[hydrocarbon]]) to produce carbon dioxide and water, activation energy is supplied in the form of heat or a spark. In the case of photochemical reactions, light provides the activation energy. The absorption of light by a reactant elevates the reactant to a higher energy state, or [[excited state]], and the process is called "[[photoexcitation]]." | ||
| + | |||
| + | The absorption of a [[photon]] of light by a reactant molecule may permit a reaction to occur not just by bringing the molecule to the necessary activation energy, but also by changing the symmetry of the molecule's electronic configuration, enabling an otherwise inaccessible reaction path. | ||
| + | |||
| + | A substance that absorbs radiation and transfers energy to the reactant is called a "[[photosensitizer]]." When a photoexcited state is deactivated by a chemical reagent, the process is called "[[quenching]]." | ||
| + | |||
| + | == Laws of photochemistry == | ||
The first law of photochemistry, known as the [[Grotthuss-Draper law]] (for chemists [[Theodor Grotthuss]] and [[John W. Draper]]), states that light must be absorbed by a chemical substance in order for a [[photochemical reaction]] to take place. | The first law of photochemistry, known as the [[Grotthuss-Draper law]] (for chemists [[Theodor Grotthuss]] and [[John W. Draper]]), states that light must be absorbed by a chemical substance in order for a [[photochemical reaction]] to take place. | ||
| Line 7: | Line 18: | ||
The second law of photochemistry, the [[Stark-Einstein law]], states that for each photon of light absorbed by a chemical system, only one molecule is activated for a photochemical reaction. This is also known as the [[photoequivalence law]] and was derived by [[Albert Einstein]] at the time when the [[quantum mechanics|quantum (photon) theory]] of light was being developed. | The second law of photochemistry, the [[Stark-Einstein law]], states that for each photon of light absorbed by a chemical system, only one molecule is activated for a photochemical reaction. This is also known as the [[photoequivalence law]] and was derived by [[Albert Einstein]] at the time when the [[quantum mechanics|quantum (photon) theory]] of light was being developed. | ||
| − | + | ==Regions of the electromagnetic spectrum== | |
| − | The | + | The [[electromagnetic spectrum]] is broad, but photochemists find themselves working with several key regions: |
| + | * Visible Light: 400–700 nanometer (nm) wavelength range | ||
| + | * Ultraviolet: 100–400 nm wavelength range | ||
| + | * Near Infrared: 700–1000 nm wavelength range | ||
| + | * Far infrared: 15–1000 micrometer (µm) wavelength range | ||
| − | == | + | == Units and constants == |
| − | |||
| − | + | Like most scientific disciplines, photochemistry utilizes the SI, or metric, measurement system. Important units and constants that show up regularly include the [[meter]] (and variants such as centimeter, millimeter, micrometer, and nanometer), seconds, hertz, joules, [[mole (chemistry)|moles]], the [[gas constant]] ''R,'' and the [[Boltzmann constant]]. These units and constants are also integral to the field of [[physical chemistry]]. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | == Notes == |
| − | + | <references/> | |
| − | |||
==References == | ==References == | ||
| − | + | * Coyle, John D. 1991. ''Introduction to Organic Photochemistry''. New York: Wiley. ISBN 0471909750 | |
| + | * Montalti, Marco, Alberto Credi, Luca Prodi, and M. Teresa Gandolfi. 2006. ''Handbook of Photochemistry,'' 3rd ed. Boca Raton, Florida: CRC Press. ISBN 0824723775 | ||
| + | * Wayne, Carol E. and Richard P. Wayne. 1996. ''Photochemistry''. Oxford: Oxford University Press. ISBN 0198558864 | ||
| + | |||
| + | == External links == | ||
| + | All links retrieved November 23, 2022. | ||
| + | * [http://www.meta-synthesis.com/webbook/17_photo/photo.html Photochemistry.] ''The Chemogenesis Web Book''. | ||
{{BranchesofChemistry}} | {{BranchesofChemistry}} | ||
| Line 33: | Line 47: | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
| − | {{ | + | {{credits|Photochemistry|146973909}} |
Latest revision as of 04:25, 24 November 2022
Photochemistry, a sub-discipline of chemistry, is the study of the interactions between atoms, molecules, and light (or electromagnetic radiation).[1] The chemical reactions that take place through these interactions are known as photochemical reactions. Examples of photochemical reactions are photosynthesis in plant cells and light-induced changes that take place in the eye. In addition, photochemical reactions are important in photography, dye bleaching, and television displays.
Reactions activated by light
A photochemical reaction may be thought of as a reaction ignited by the absorption of light. Normally, a reaction (not just a photochemical reaction) occurs when the molecules involved gain the activation energy necessary to undergo change. For example, for the combustion of gasoline (a hydrocarbon) to produce carbon dioxide and water, activation energy is supplied in the form of heat or a spark. In the case of photochemical reactions, light provides the activation energy. The absorption of light by a reactant elevates the reactant to a higher energy state, or excited state, and the process is called "photoexcitation."
The absorption of a photon of light by a reactant molecule may permit a reaction to occur not just by bringing the molecule to the necessary activation energy, but also by changing the symmetry of the molecule's electronic configuration, enabling an otherwise inaccessible reaction path.
A substance that absorbs radiation and transfers energy to the reactant is called a "photosensitizer." When a photoexcited state is deactivated by a chemical reagent, the process is called "quenching."
Laws of photochemistry
The first law of photochemistry, known as the Grotthuss-Draper law (for chemists Theodor Grotthuss and John W. Draper), states that light must be absorbed by a chemical substance in order for a photochemical reaction to take place.
The second law of photochemistry, the Stark-Einstein law, states that for each photon of light absorbed by a chemical system, only one molecule is activated for a photochemical reaction. This is also known as the photoequivalence law and was derived by Albert Einstein at the time when the quantum (photon) theory of light was being developed.
Regions of the electromagnetic spectrum
The electromagnetic spectrum is broad, but photochemists find themselves working with several key regions:
- Visible Light: 400–700 nanometer (nm) wavelength range
- Ultraviolet: 100–400 nm wavelength range
- Near Infrared: 700–1000 nm wavelength range
- Far infrared: 15–1000 micrometer (µm) wavelength range
Units and constants
Like most scientific disciplines, photochemistry utilizes the SI, or metric, measurement system. Important units and constants that show up regularly include the meter (and variants such as centimeter, millimeter, micrometer, and nanometer), seconds, hertz, joules, moles, the gas constant R, and the Boltzmann constant. These units and constants are also integral to the field of physical chemistry.
Notes
- ↑ International Union of Pure and Applied Chemistry. "photochemistry". Compendium of Chemical Terminology Internet edition. Retrieved August 21, 2007.
ReferencesISBN links support NWE through referral fees
- Coyle, John D. 1991. Introduction to Organic Photochemistry. New York: Wiley. ISBN 0471909750
- Montalti, Marco, Alberto Credi, Luca Prodi, and M. Teresa Gandolfi. 2006. Handbook of Photochemistry, 3rd ed. Boca Raton, Florida: CRC Press. ISBN 0824723775
- Wayne, Carol E. and Richard P. Wayne. 1996. Photochemistry. Oxford: Oxford University Press. ISBN 0198558864
External links
All links retrieved November 23, 2022.
- Photochemistry. The Chemogenesis Web Book.
| ||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.