Thomson, J. J.
(→External links: Checked links, removed bad link, added Retrieved dates) |
|||
| Line 68: | Line 68: | ||
==References== | ==References== | ||
| − | |||
| − | * Dahl, Per F. | + | * Dahl, Per F. ''Flash of the Cathode Rays: A History of J.J. Thomson's Electron''. Philadelphia, PA: Institute of Physics Publishing. ISBN 0-7503-0453-7. |
| − | * | + | * Thomson, J.J. 1897. [http://web.lemoyne.edu/~GIUNTA/thomson1897.html Cathode rays]. ''Philosophical Magazine''. 44:293. Retrieved December 12, 2007. |
| − | * | + | * Thomson, J.J. 1913. [http://web.lemoyne.edu/~giunta/canal.html Rays of positive electricity.] ''Proceedings of the Royal Society''. A89:1-20. Retrieved December 12, 2007. |
| − | *[http://dbhs.wvusd.k12.ca.us/webdocs/Chem-History/Thomson-Structure-Atom.html | + | * Thomson, J.J. 1904. [http://dbhs.wvusd.k12.ca.us/webdocs/Chem-History/Thomson-Structure-Atom.html On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure]. ''Philosophical Magazine''. 6:7:39. Retrieved December 12, 2007. |
| − | *[http://www.trin.cam.ac.uk/index.php?pageid=172 The Master of Trinity] | + | *[http://www.trin.cam.ac.uk/index.php?pageid=172 The Master of Trinity]. Trinity College. Retrieved December 12, 2007. |
==External links== | ==External links== | ||
| − | * [http://www.aip.org/history/electron/ The Discovery of the Electron] | + | * [http://www.aip.org/history/electron/ The Discovery of the Electron]. Retrieved December 12, 2007. |
| − | * [http://alsos.wlu.edu/qsearch.aspx?browse=people/Thomson,+Joseph+J. Annotated bibliography for Joseph J. Thomson from the Alsos Digital Library] | + | * [http://alsos.wlu.edu/qsearch.aspx?browse=people/Thomson,+Joseph+J. Annotated bibliography for Joseph J. Thomson from the Alsos Digital Library]. Retrieved December 12, 2007. |
| − | * [http://www.asa3.org/asa/PSCF/1986/JASA6-86Seeger.html Essay on Thomson life and religious views] | + | * [http://www.asa3.org/asa/PSCF/1986/JASA6-86Seeger.html Essay on Thomson life and religious views]. Retrieved December 12, 2007. |
| − | *[http://members.chello.nl/~h.dijkstra19/page3.html The Cathode Ray Tube site] | + | *[http://members.chello.nl/~h.dijkstra19/page3.html The Cathode Ray Tube site]. Retrieved December 12, 2007. |
| − | *[http://dlxs2.library.cornell.edu/cgi/t/text/text-idx?c=cdl;idno=cdl022 Notes on recent researches in electricity and magnetism] Cornell University Library Historical Monographs Collection. | + | *[http://dlxs2.library.cornell.edu/cgi/t/text/text-idx?c=cdl;idno=cdl022 Notes on recent researches in electricity and magnetism] Cornell University Library Historical Monographs Collection. Retrieved December 12, 2007. |
{{Nobel Prize in Physics Laureates 1901-1925}} | {{Nobel Prize in Physics Laureates 1901-1925}} | ||
Revision as of 19:10, 12 December 2007
|
Sir Joseph John Thomson | |
|---|---|
 | |
| Born |
18 December 1856 |
| Died | 30 August 1940 (aged 83) Cambridge, UK |
| Residence | United Kingdom |
| Nationality | United Kingdom |
| Field | Physicist |
| Institutions | University of Cambridge Princeton University Yale University |
| Alma mater | University of Manchester University of Cambridge |
| Academic advisor | John Strutt (Rayleigh) Edward John Routh |
| Notable students | Charles T. R. Wilson Ernest Rutherford Francis William Aston John Townsend Owen Richardson William Henry Bragg Harold A. Wilson H. Stanley Allen |
| Known for | Plum pudding model Discovery of electron Discovery of isotopes Invention of the mass spectrometer |
| Notable prizes | |
| Religious stance | Anglican |
| Thomson is the father of Nobel laureate George Paget Thomson. | |
Sir Joseph John “J.J.” Thomson, OM, FRS (18 December 1856 – 30 August 1940) was a British physicist and Nobel laureate, credited for the discovery of the electron and of isotopes, and the invention of the mass spectrometer. He was awarded the 1906 Nobel Prize in Physics for the discovery of the electron.
Life
J.J. Thomson was born in 1856 in Cheetham Hill, Manchester in England, of Scottish parentage. In 1870 he studied engineering at University of Manchester known as Owens College at that time, and moved on to Trinity College, Cambridge in 1876. In 1880, he obtained his BA in mathematics (Second Wrangler and 2nd Smith's prize) and MA (with Adams Prize) in 1883. In 1884 he became Cavendish Professor of Physics. One of his students was Ernest Rutherford, who would later succeed him in the post. He rejected his suitor Rachel Love which left her heartbroken, but in 1890 he married Rose Elisabeth Paget, daughter of Sir George Edward Paget, KCB, a physician and then Regius Professor of Physic at Cambridge. He fathered one son, George Paget Thomson, and one daughter, Joan Paget Thomson, with her. His son became a noted physicist in his own right, winning the Nobel Prize himself for proving the wavelike properties of electrons.
He was awarded a Nobel Prize in 1906, "in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases." He was knighted in 1908 and appointed to the Order of Merit in 1912. In 1914 he gave the Romanes Lecture in Oxford on "The atomic theory." In 1918 he became Master of Trinity College, Cambridge, where he remained until his death. He died on August 30, 1940 and was buried in Westminster Abbey, close to Sir Isaac Newton.
Thomson was elected to Fellow of the Royal Society on June 12, 1884 and was subsequently the president of the Royal Society from 1916 to 1920.then died in 1940
Work on cathode rays
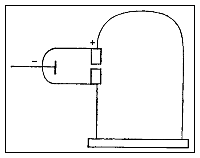
Thomson conducted a series of experiments with cathode rays and cathode ray tubes leading him to the discovery of electrons and subatomic particles. Thomson used the cathode ray tube in three different experiments.
First Experiment
In his first experiment, he investigated whether or not the negative charge could be separated from the cathode rays by means of magnetism. He constructed a cathode ray tube ending in a pair of cylinders with slits in them. These slits were in turn connected to an electrometer. Thomson found that if the rays were magnetically bent such that they could not enter the slit, the electrometer registered little charge. Thomson concluded that the negative charge was inseparable from the rays.
Second experiment
In his second experiment, he investigated whether or not the rays could be deflected by an electric field (something that is characteristic of charged particles). Previous experimenters had failed to observe this, but Thomson believed their experiments were flawed because they contained trace amounts of gas. Thomson constructed a cathode ray tube with a practically perfect vacuum, and coated one end with phosphorescent paint. Thomson found that the rays did indeed bend under the influence of an electric field.
Third experiment
In his third experiment, Thomson measured the charge-to-mass ratio of the cathode rays by measuring how much they were deflected by a magnetic field and how much energy they carried. He found that the charge to mass ratio was over a thousand times higher than that of a proton, suggesting either that the particles were very light or very highly charged.
Thomson's conclusions were bold: cathode rays were indeed made of particles which he called "corpuscles," and these corpuscles came from within the atoms of the electrodes themselves, meaning they were in fact divisible. Thomson imagined the atom as being made up of these corpuscles swarming in a sea of positive charge; this was his plum pudding model. This model was later proved incorrect by Ernest Rutherford.
His discovery was made known in 1897, and caused a sensation in scientific circles, eventually resulting in him being awarded a Nobel Prize in Physics in 1906.
Discovery of isotopes
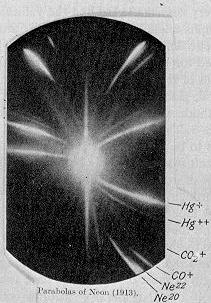
In 1913, as part of his exploration into the composition of canal rays, Thomson channeled a stream of ionized neon through a magnetic and an electric field and measured its deflection by placing a photographic plate in its path. Thomson observed two patches of light on the photographic plate (see image on right), which suggested two different parabolas of deflection. Thomson concluded that the neon gas was composed of atoms of two different atomic masses (neon-20 and neon-22).
Other work
In 1906, Thomson demonstrated that hydrogen had only a single electron. Previous theories allowed various numbers of electrons.[1]
Awards
- Royal Medal (1894)
- Hughes Medal (1902)
- Nobel Prize for Physics (1906)
- Copley Medal (1914)
Notes
- ↑ Hellemans, Alexander, and Bryan H. Bunch. 1988. The Timetables of Science: A Chronology of the Most Important People and Events in the History of Science. New York: Simon and Schuster, 411. ISBN 0671621300.
ReferencesISBN links support NWE through referral fees
- Dahl, Per F. Flash of the Cathode Rays: A History of J.J. Thomson's Electron. Philadelphia, PA: Institute of Physics Publishing. ISBN 0-7503-0453-7.
- Thomson, J.J. 1897. Cathode rays. Philosophical Magazine. 44:293. Retrieved December 12, 2007.
- Thomson, J.J. 1913. Rays of positive electricity. Proceedings of the Royal Society. A89:1-20. Retrieved December 12, 2007.
- Thomson, J.J. 1904. On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure. Philosophical Magazine. 6:7:39. Retrieved December 12, 2007.
- The Master of Trinity. Trinity College. Retrieved December 12, 2007.
External links
- The Discovery of the Electron. Retrieved December 12, 2007.
- Annotated bibliography for Joseph J. Thomson from the Alsos Digital Library. Retrieved December 12, 2007.
- Essay on Thomson life and religious views. Retrieved December 12, 2007.
- The Cathode Ray Tube site. Retrieved December 12, 2007.
- Notes on recent researches in electricity and magnetism Cornell University Library Historical Monographs Collection. Retrieved December 12, 2007.
| ||||||||
| Persondata | |
|---|---|
| NAME | Thomson, Joseph John |
| ALTERNATIVE NAMES | |
| SHORT DESCRIPTION | English physicist |
| DATE OF BIRTH | 18 December 1856 |
| PLACE OF BIRTH | Cheetham Hill, Manchester |
| DATE OF DEATH | 30 August 1940 |
| PLACE OF DEATH | Cambridge |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.