Fullerene
The fullerenes are recently-discovered allotropes of carbon. They are molecules composed entirely of carbon, which take the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are sometimes called buckyballs, while cylindrical fullerenes are called buckytubes or nanotubes.
Naming
Buckminsterfullerene (C60) was named for Richard Buckminster Fuller, a noted architect who popularized the geodesic dome. Since buckminsterfullerenes have a similar shape to that sort of dome, the name was thought to be appropriate.
Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
Buckminsterfullerene
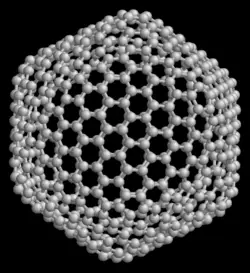
The smallest fullerene in which no two pentagons share an edge (which is destabilizing — see pentalene) is C60 (buckminsterfullerene), and this is also the most common.
The structure of C60 is that of a truncated icosahedron, which resembles a round soccer ball of the type made of hexagons and pentagons, with a carbon atom at the corners of each hexagon and a bond along each edge. A polymerized single-walled nanotubule (P-SWNT) is a substance composed of polymerized fullerenes in which carbon atoms from one buckytube bond with carbons in other buckytubes.
Prediction and discovery
In molecular beam experiments, discrete peaks were observed corresponding to molecules with the exact mass of 60, 70, or greater numbers of carbon atoms. Harold Kroto, from the University of Sussex, James Heath, Sean O'Brien, Robert Curl and Richard Smalley, from Rice University, discovered C60 and the fullerenes in 1985. Kroto, Curl, and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of this class of compounds. C60 and other fullerenes were later noticed occurring outside of a laboratory environment (e.g. in normal candle soot). By 1991, it was relatively easy to produce grams of fullerene powder using the techniques of Donald Huffman and Wolfgang Krätschmer. Fullerene purification remains a challenge to chemists and determines fullerene prices to a large extent. So called endohedral fullerenes have ions or small molecules incorporated inside the cage atoms. Fullerene is an unusual reactant in many organic reactions such as the Bingel reaction discovered in 1993.
Properties
As of the early twenty-first century, the chemical and physical properties of fullerenes are still under heavy study, in both pure and applied research labs. In April 2003, fullerenes were under study for potential medicinal use — binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma. In the October 2005 issue of Chemistry and Biology, an article [1] describing the use of fullerenes as light-activated antimicrobial agents was published.
Fullerenes are not very reactive due to the stability of the graphite-like bonds, and are also sparingly soluble in many solvents. Common solvents for the fullerenes include toluene and carbon disulfide. Solutions of pure Buckminsterfullerene have a deep purple color. Fullerenes are the only known allotrope of carbon that can be dissolved. Researchers have been able to increase the reactivity by attaching active groups to the surfaces of fullerenes. Buckminsterfullerene does not exhibit "superaromaticity". That is, the electrons in the hexagonal rings do not delocalize over the whole molecule.
Other atoms can be trapped inside fullerenes, and indeed recent evidence for a meteor impact at the end of the Permian period was found by analysing noble gases so preserved.
In the field of nanotechnology, heat resistance and superconductivity are some of the more heavily studied properties.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
Possible dangers
Although buckyballs have been thought in theory to be relatively inert, a presentation given to the American Chemical Society in March 2004 and described in an article in New Scientist on April 3 2004, suggests the molecule is injurious to organisms. An experiment by Eva Oberdörster at Southern Methodist University, which introduced fullerenes into water at concentrations of 0.5 parts per million, found that largemouth bass suffered a 17-fold increase in cellular damage in the brain tissue after 48 hours. The damage was of the type lipid peroxidation, which is known to impair the functioning of cell membranes. There were also inflammatory changes in the liver and activation of genes related to the making of repair enzymes. At the time of presentation, the SMU work had not been peer reviewed.
Because of their notable properties, buckyballs may be part of many products in the near future. With this knowledge in mind, many researchers are investigating ways to reduce the toxicity of the buckyballs [2]. They have discovered that they can reduce the toxicity by adding hydroxyls, among other chemical groups. With each chemical group added to the buckyball, the scientists could reduce its toxicity level by an order of magnitude.
It is believed that buckyballs acquire their toxicity by producing free radicals in water that damage the lipids on the cellular membranes of animals, destroying the cells.
Pristine C60 can be suspended in water at low concentrations as large clusters often termed nC60. These clusters are spherical clumps of C60 between 250-350 nm in diameter. Thus, nC60 represents a different chemical entity than solutions of C60 in which the fullerenes exist as individual molecules. Recently, results presented at the ACS meeting in Anaheim, CA suggest that nC60 is moderately toxic to water fleas and juvenile largemouth bass at concentrations in water of around 800 ppb. The first study of its kind on marine life, these preliminary results quickly spread across the scientific community. However, the overwhelming evidence of the essential non-toxicity of C60 (not nC60) in previously peer-reviewed articles of C60 and many of its derivatives indicates that our compounds are likely to have little (if any) toxicity, especially at the very low concentration at which it is used (~1-10 µM).
Fullerene extract mixture (C60/C70) solubility
Solvents that dissolve fullerenes are listed below in order from highest solubility. The value in parentheses is the approximate saturated concentration.
- 1,2,4-trichlorobenzene (20mg/ml)
- carbon disulfide (12mg/ml)
- toluene (3.2mg/ml)
- benzene (1.8mg/ml)
- chloroform (0.5mg/ml)
- carbon tetrachloride (0.4mg/ml)
- cyclohexane (0.054mg/ml)
- n-hexane (0.046mg/ml)
- THF (0.037mg/ml)
- acetonitrile (0.02mg/ml)
- methanol (0.0009mg/ml)
Diffraction of fullerene
In 1999, researchers from the University of Vienna Template:An demonstrated that the wave-particle duality applied to macro-molecules such as fullerene.
Notes
- Template:Anb Wave-particle duality of C60, M. Arndt , O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, A. Zeilinger, Nature 401, 680-682, 14 October 1999
Mathematics of fullerenes
In mathematical terms, the structure of a fullerene is a trivalent convex polyhedron with pentagonal and hexagonal faces. Using Euler formula F - E + V = 2, (plus the fact that every vertex in a fullerene structure belongs to exactly 3 faces) one can easily prove that there are exactly 12 pentagons in a fullerene. The smallest fullerene is C20, the dodecahedron. There are no fullerenes with 22 vertices. The number of fullerenes C2n grows rapidly with increasing n = 12,13, ... For instance, there are 1812 non-isomorphic fullerenes C60 but only one of them, the buckminsterfullerene alias truncated icosahedron, has no pair of adjacent pentagons.
Media
|
|
Further reading
- The Most Beautiful Molecule: The Discovery of the Buckyball by Hugh Aldersey-Williams (John Wiley & Sons, 1995) ISBN 0-471-19333-X
See also
- Carbon nanotube
- Dodecahedrane
- Graphene
- Polyhedron
- Geodesic dome
- Prismane C8
External links
- Kim Allen
- Center for Nanoscale Science and Technology
- Dr. Smalley's brief autobiography
- Dr. Smalley's webpage
- Potential use of fullerenes in medicine
- Carbon Fullerene & Nanotube Models Vincent Herr, Houston, TX
- Fullerene Images for Web and Presentation
- Discovery of graphene
- Diffraction and Interference with Fullerenes: Wave-particle duality of C60, University of Vienna
- Fullerene-based architectures for quantum computing in Germany and in Great Britain at the QIP IRC
- Molview from bluerhinos.co.uk See Buckminsterfullerene (C60) in 3D
- Interactive 3D molecular visualization of fullerene (requires Macromedia Flash)
- Computational Chemistry Wiki
cs:Fulleren de:Fulleren es:Fulereno eo:Fulereno fr:Fullerène it:Fullerene he:פולרין nl:Fullereen ja:フラーレン pl:Fuleren pt:Buckminsterfullerenos ro:Fulerene ru:Фуллерены su:Fullerin fi:Fullereeni sv:Fulleren zh:富勒烯
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.