Difference between revisions of "Fullerene" - New World Encyclopedia
m |
|||
| Line 29: | Line 29: | ||
===Boron buckyball=== | ===Boron buckyball=== | ||
| − | A new type of buckyball utilizing [[boron]] atoms instead of the usual carbon has been predicted and described by researchers at Rice University. The B-80 structure is predicted to be more stable than the C-60 buckyball. <ref> | + | A new type of buckyball utilizing [[boron]] atoms instead of the usual carbon has been predicted and described by researchers at Rice University. The B-80 structure is predicted to be more stable than the C-60 buckyball. <ref>Boyd, Jade. 2007. [http://www.eurekalert.org/pub_releases/2007-04/ru-bbt042307.php Bucky's brother—The boron buckyball makes its debut]. eurekalert.org. Retrieved December 4, 2007.</ref> One reason for this given by the researchers is that the B-80 is actually more like the original geodesic dome structure popularized by Buckminster Fuller which utilizes triangles rather than hexagons. |
===Variations of buckballs=== | ===Variations of buckballs=== | ||
| − | Another fairly common buckminsterfullerene is [[C70|C<sub>70</sub>]],<ref>[http://www.bristol.ac.uk/Depts/Chemistry/MOTM/buckyball/c60a.htm]</ref> but fullerenes with 72 and even up to 100 carbon atoms are commonly obtained. | + | Another fairly common buckminsterfullerene is [[C70|C<sub>70</sub>]],<ref>[http://www.bristol.ac.uk/Depts/Chemistry/MOTM/buckyball/c60a.htm Buckminsterfullerene, C<SUB>60</SUB>]. University of Bristol. Retrieved December 4, 2007.</ref> but fullerenes with 72 and even up to 100 carbon atoms are commonly obtained. |
In [[mathematics|mathematical]] terms, the structure of a '''fullerene''' is a [[Valence (chemistry)|trivalent]] convex [[polyhedron]] with pentagonal and hexagonal faces. In [[graph theory]], the term '''fullerene''' refers to any 3-[[Regular graph|regular]], [[Planar graph|planar]] graph with all faces of size 5 or 6 (including the external face). It follows from [[Euler characteristic|Euler's polyhedron formula]], |V|-|E|+|F| = 2, (where |V|, |E|, |F| indicate the number of vertices, edges, and faces), that there are exactly 12 pentagons in a fullerene and |V|/2-10 hexagons. | In [[mathematics|mathematical]] terms, the structure of a '''fullerene''' is a [[Valence (chemistry)|trivalent]] convex [[polyhedron]] with pentagonal and hexagonal faces. In [[graph theory]], the term '''fullerene''' refers to any 3-[[Regular graph|regular]], [[Planar graph|planar]] graph with all faces of size 5 or 6 (including the external face). It follows from [[Euler characteristic|Euler's polyhedron formula]], |V|-|E|+|F| = 2, (where |V|, |E|, |F| indicate the number of vertices, edges, and faces), that there are exactly 12 pentagons in a fullerene and |V|/2-10 hexagons. | ||
| Line 50: | Line 50: | ||
==Properties== | ==Properties== | ||
| − | For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. [[Popular Science]] has published articles about the possible uses of fullerenes in [[armor]].{{Fact|date=February 2007}} In April 2003, fullerenes were under study for [[Nanomedicine|potential medicinal use]]: binding specific [[antibiotic]]s to the structure to target resistant [[bacterium|bacteria]] and even target certain [[cancer]] cells such as [[melanoma]]. The October 2005 issue of [[Chemistry and Biology]] contains an article describing the use of fullerenes as light-activated [[antimicrobial]] agents.<ref> | + | For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. [[Popular Science]] has published articles about the possible uses of fullerenes in [[armor]].{{Fact|date=February 2007}} In April 2003, fullerenes were under study for [[Nanomedicine|potential medicinal use]]: binding specific [[antibiotic]]s to the structure to target resistant [[bacterium|bacteria]] and even target certain [[cancer]] cells such as [[melanoma]]. The October 2005 issue of [[Chemistry and Biology]] contains an article describing the use of fullerenes as light-activated [[antimicrobial]] agents.<ref>Tegos, G., T. Demidova, D. Arcila-Lopez, H. Lee, T. Wharton, H. Gali, M. Hamblin. 2005. Cationic Fullerenes Are Effective and Selective Antimicrobial Photosensitizers. ''Chemistry & Biology''. 12:10:1127-1135. Retrieved December 4, 2007.</ref> |
In the field of [[nanotechnology]], heat resistance and [[superconductivity]] are some of the more heavily studied properties. | In the field of [[nanotechnology]], heat resistance and [[superconductivity]] are some of the more heavily studied properties. | ||
| Line 67: | Line 67: | ||
Fullerenes are stable, but not totally nonreactive. The sp<sup>2</sup>-hybridized carbon atoms, which are at their energy minimum in planar graphite, must be bent to form the closed sphere or tube, which produces [[angle strain]]. The characteristic reaction of fullerenes is [[electrophilic addition]] at 6,6-double bonds, which reduces angle strain by changing sp<sup>2</sup>-hybridized carbons into sp<sup>3</sup>-hybridized ones. The change in hybridized orbitals causes the bond angles to decrease from about 120 degrees in the sp<sup>2</sup> orbitals to about 109.5 degrees in the sp<sup>3</sup> orbitals. This decrease in bond angles allows for the bonds to bend less when closing the sphere or tube, and thus, the molecule becomes more stable. | Fullerenes are stable, but not totally nonreactive. The sp<sup>2</sup>-hybridized carbon atoms, which are at their energy minimum in planar graphite, must be bent to form the closed sphere or tube, which produces [[angle strain]]. The characteristic reaction of fullerenes is [[electrophilic addition]] at 6,6-double bonds, which reduces angle strain by changing sp<sup>2</sup>-hybridized carbons into sp<sup>3</sup>-hybridized ones. The change in hybridized orbitals causes the bond angles to decrease from about 120 degrees in the sp<sup>2</sup> orbitals to about 109.5 degrees in the sp<sup>3</sup> orbitals. This decrease in bond angles allows for the bonds to bend less when closing the sphere or tube, and thus, the molecule becomes more stable. | ||
| − | Other atoms can be trapped inside fullerenes to form [[inclusion compound]]s known as [[endohedral fullerenes]]. An unusual example is the egg shaped fullerene Tb<sub>3</sub>N@C<sub>84</sub>, which violates the isolated pentagon rule.<ref> | + | Other atoms can be trapped inside fullerenes to form [[inclusion compound]]s known as [[endohedral fullerenes]]. An unusual example is the egg shaped fullerene Tb<sub>3</sub>N@C<sub>84</sub>, which violates the isolated pentagon rule.<ref>Beavers, Christine M., Tianming Zuo, James C. Duchamp, Kim Harich, Harry C. Dorn, Marilyn M. Olmstead, and Alan L. Balch. 2006. [http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/2006/128/i35/abs/ja063636k.html Tb<sub>3</sub>N@C<sub>84</sub>: An Improbable, Egg-Shaped Endohedral Fullerene that Violates the Isolated Pentagon Rule]. ''J. Am. Chem. Soc.'' 128:35:11352-11353. Retrieved December 4, 2007.</ref> Recent evidence for a meteor impact at the end of the [[Permian]] period was found by analysing [[noble gas]]es so preserved.<ref>Becker, Luann, Robert J. Poreda, Andrew G. Hunt, Theodore E. Bunch, Michael Rampino. 2007. [http://sciencemag.org/cgi/content/abstract/291/5508/1530 Impact Event at the Permian-Triassic Boundary: Evidence from Extraterrestrial Noble Gases in Fullerenes]. ''Science''. 291:5508:1530-3. Retrieved December 4, 2007.</ref> Metallofullerene-based inoculates using the rhonditic steel process are beginning production as one of the first commercially-viable uses of buckyballs. <!-- What does this mean? Can we get some links? —> |
===Solubility=== | ===Solubility=== | ||
| Line 73: | Line 73: | ||
Fullerenes are sparingly soluble in many [[solvent]]s. Common solvents for the fullerenes include aromatics, such as [[toluene]], and others like [[carbon disulfide]]. Solutions of pure Buckminsterfullerene have a deep purple color. Solutions of C<sub>70</sub> are a reddish brown. The higher fullerenes C<sub>76</sub> to C<sub>84</sub> have a variety of colors. C<sub>76</sub> has two optical forms, while other higher fullerenes have several structural isomers. Fullerenes are the only known [[allotrope]] of carbon that can be dissolved in common solvents at room temperature. | Fullerenes are sparingly soluble in many [[solvent]]s. Common solvents for the fullerenes include aromatics, such as [[toluene]], and others like [[carbon disulfide]]. Solutions of pure Buckminsterfullerene have a deep purple color. Solutions of C<sub>70</sub> are a reddish brown. The higher fullerenes C<sub>76</sub> to C<sub>84</sub> have a variety of colors. C<sub>76</sub> has two optical forms, while other higher fullerenes have several structural isomers. Fullerenes are the only known [[allotrope]] of carbon that can be dissolved in common solvents at room temperature. | ||
| − | Some fullerene structures are not soluble because they have a small bandgap between the ground and excited states. These include the small fullerenes C<sub>28</sub><ref>[http://adsabs.harvard.edu/abs/1993JChPh..99..352G Ab initio theoretical predictions of C28, C28H4, C28F4, (Ti at C28)H4, and M at C28 (M = Mg, Al, Si, S, Ca, Sc, Ti, Ge, Zr, and Sn)] | + | Some fullerene structures are not soluble because they have a small bandgap between the ground and excited states. These include the small fullerenes C<sub>28</sub><ref>Guo, Ting, Richard E. Smalley, Gustavo E. Scuseria. 1993. [http://adsabs.harvard.edu/abs/1993JChPh..99..352G Ab initio theoretical predictions of C28, C28H4, C28F4, (Ti at C28)H4, and M at C28 (M = Mg, Al, Si, S, Ca, Sc, Ti, Ge, Zr, and Sn)]. ''Journal of Chemical Physics''. 99:1:352-359. Retrieved December 4, 2007.</ref>, C<sub>36</sub> and C<sub>50</sub>. The C<sub>72</sub> structure is also in this class, but the endohedral version with a trapped lanthanide-group atom is soluble due to the interaction of the metal atom and the electronic states of the fullerene. Researchers had originally been puzzled by C<sub>72</sub> being absent in fullerene plasma-generated soot extract, but found in endohedral samples. Small band gap fullerenes are highly reactive and bind to other fullerenes or to soot particles. |
| − | Solvents that are able to dissolve buckminsterfullerene (C<sub>60</sub>) are listed below in order from highest solubility. The value in parentheses is the approximate saturated concentration.<ref> | + | Solvents that are able to dissolve buckminsterfullerene (C<sub>60</sub>) are listed below in order from highest solubility. The value in parentheses is the approximate saturated concentration.<ref>Bezmel'nitsyn, V.N., A.V. Eletskiĭ, M.V. Okun'. 1998. Fullerenes in Solutions. ''Uspekhi Fizicheskikh Nauk'', Russian Academy of Sciences, 41.</ref> |
# [[1-chloronaphthalene]] (51 mg/mL) | # [[1-chloronaphthalene]] (51 mg/mL) | ||
# [[1-methylnaphthalene]] (33 mg/mL) | # [[1-methylnaphthalene]] (33 mg/mL) | ||
| Line 97: | Line 97: | ||
Solubility of C<sub>60</sub> in some solvents shows unusual behavior due to existance of solvate phases (analogues of crystallohydrates). For example, solubility of C60 in beznene solution shows maximum at about 313K. Crystallization from benzene solution at temperatures below maximum results in formation of triclinic solid solvate with four benzene molecules C<sub>60</sub>*4C<sub>6</sub>H<sub>6</sub> which is rather unstable on air. Out of solution this structure decomposes into usual fcc C<sub>60</sub> in few minutes time. At temperatures above solubility maximum the solvate is not stable even when immersed in saturated solution and melts with formation of fcc C60. Crystallization at temperatures above the solubility maximum results in formation of pure fcc C60. Large millimeter size crystals of C<sub>60</sub> and C<sub>70</sub> can be grown from solution both for solvates and for pure fullerenes. | Solubility of C<sub>60</sub> in some solvents shows unusual behavior due to existance of solvate phases (analogues of crystallohydrates). For example, solubility of C60 in beznene solution shows maximum at about 313K. Crystallization from benzene solution at temperatures below maximum results in formation of triclinic solid solvate with four benzene molecules C<sub>60</sub>*4C<sub>6</sub>H<sub>6</sub> which is rather unstable on air. Out of solution this structure decomposes into usual fcc C<sub>60</sub> in few minutes time. At temperatures above solubility maximum the solvate is not stable even when immersed in saturated solution and melts with formation of fcc C60. Crystallization at temperatures above the solubility maximum results in formation of pure fcc C60. Large millimeter size crystals of C<sub>60</sub> and C<sub>70</sub> can be grown from solution both for solvates and for pure fullerenes. | ||
| − | <ref>Talyzin A.V. | + | <ref>Talyzin A.V. 1997. Phase transition C60-C60*4C6H6 in liquid benzene. ''J. of Phys.Chem''. 101:47.</ref> |
| − | <ref>Talyzin A.V., Engstrцm | + | <ref>Talyzin A.V., I. Engstrцm. 1998. C70 in a Benzene, Hexane and Toluene solutions. ''J. of Phys.Chem''. 102:34:6477-6481.</ref> |
=== Quantum mechanics === | === Quantum mechanics === | ||
| − | In 1999, researchers from the University of Vienna demonstrated that the [[wave-particle duality]] applied to molecules such as fullerene<ref> | + | In 1999, researchers from the University of Vienna demonstrated that the [[wave-particle duality]] applied to molecules such as fullerene<ref>Arndt, M., O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, Anton Zeilinger. 1999. Wave-particle duality of C60. ''Nature''. 401:680-682.</ref>. One of the co-authors of this research, [[Julian Voss-Andreae]] became an artist and has since created several sculptures [[fullerenes in popular culture|symbolizing wave-particle duality in Buckminsterfullerenes]]. |
| − | Science writer Marcus Chown made a reference on the CBC radio show "Quirks And Quarks" in May 2006 that there is a scientist working on having buckyballs follow the quantum behavior of atoms of appearing to be in two places at once. The work is continuing on this phenomenon | + | Science writer Marcus Chown made a reference on the CBC radio show "Quirks And Quarks" in May 2006 that there is a scientist working on having buckyballs follow the quantum behavior of atoms of appearing to be in two places at once. The work is continuing on this phenomenon. |
=== Safety === | === Safety === | ||
| Line 119: | Line 119: | ||
*In ''[[New Scientist]]'' there used to be a weekly column called ''Daedalus'' written by David Jones, which contained humorous descriptions of unlikely technologies. In 1966 the columnist included a description of C<sub>60</sub> and other forms of graphite. This was meant as pure entertainment. | *In ''[[New Scientist]]'' there used to be a weekly column called ''Daedalus'' written by David Jones, which contained humorous descriptions of unlikely technologies. In 1966 the columnist included a description of C<sub>60</sub> and other forms of graphite. This was meant as pure entertainment. | ||
*Also in ''New Scientist'' magazine, a free book was enclosed entitled, "100 Things to Do Before You Die," one of which was to kick a buckyball. | *Also in ''New Scientist'' magazine, a free book was enclosed entitled, "100 Things to Do Before You Die," one of which was to kick a buckyball. | ||
| − | *The buckyball is the state molecule of [[Texas]] <ref> | + | *The buckyball is the state molecule of [[Texas]] <ref>[http://www.legis.state.tx.us/tlodocs/75R/billtext/html/HC00083H.htm State molecule of Texas]. Texas Legislature Online. Retrieved December 4, 2007.</ref> |
== See also == | == See also == | ||
Revision as of 21:52, 4 December 2007
| Topics | |
|---|---|
| History · Implications Applications · Organizations Popular culture · List of topics | |
| Subfields and related fields | |
| Nanomedicine Molecular self-assembly Molecular electronics Scanning probe microscopy Nanolithography Molecular nanotechnology | |
| Nanomaterials | |
| Nanomaterials · Fullerene Carbon nanotubes Fullerene chemistry Applications · Popular culture Timeline · Carbon allotropes Nanoparticles · Quantum dots Colloidal gold · Colloidal silver | |
| Molecular nanotechnology | |
| Molecular assembler Mechanosynthesis Nanorobotics · Grey goo K. Eric Drexler Engines of Creation | |
The Fullerenes, discovered in 1985 by Robert Curl, Harold Kroto and Richard Smalley at the University of Sussex and Rice University, are a family of carbon allotropes named after Richard Buckminster Fuller and are sometimes called buckyballs. They are molecules composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Cylindrical fullerenes are called carbon nanotubes or buckytubes. Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain also pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
Prediction and discovery
In molecular beam experiments, discrete peaks were observed corresponding to molecules with the exact mass of sixty or seventy or more carbon atoms. In 1985, Harold Kroto (then of the University of Sussex, now of Florida State University), James R. Heath, Sean O'Brien, Robert Curl and Richard Smalley, from Rice University, discovered C60, and shortly thereafter came to discover the fullerenes. Kroto, Curl, and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of this class of compounds. C60 and other fullerenes were later noticed occurring outside the laboratory (e.g., in normal candle soot). By 1991, it was relatively easy to produce gram-sized samples of fullerene powder using the techniques of Donald Huffman and Wolfgang Krätschmer. Fullerene purification remains a challenge to chemists and to a large extent determines fullerene prices. So-called endohedral fullerenes have ions or small molecules incorporated inside the cage atoms. Fullerene is an unusual reactant in many organic reactions such as the Bingel reaction discovered in 1993.
The existence of C60 was predicted in 1970 by Eiji Osawa of Toyohashi University of Technology. He noticed that the structure of a corannulene molecule was a subset of a soccer-ball shape, and he made the hypothesis that a full ball shape could also exist. His idea was reported in Japanese magazines, but did not reach Europe or America.
Naming
Buckminsterfullerene (C60) was named after Richard Buckminster Fuller, a noted architect who popularized the geodesic dome. Since buckminsterfullerenes have a similar shape to that sort of dome, the name was thought to be appropriate. As the discovery of the fullerene family came after buckminsterfullerene, the name was shortened to illustrate that the latter is a type of the former.
For illustrations of geodesic dome structures, see Montreal Biosphere, Eden Project, Missouri Botanical Gardens, Science World at TELUS World of Science, Mitchell Park Horticultural Conservatory, Gold Dome, Tacoma Dome, and Spaceship Earth (Disney).
Buckyballs
Buckminsterfullerene
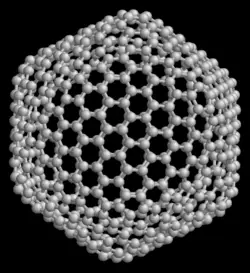
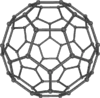
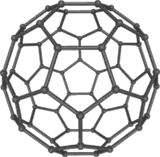
Buckminsterfullerene (IUPAC name (C60-Ih)[5,6]fullerene) is the smallest fullerene molecule in which no two pentagons share an edge (which can be destabilizing; see pentalene). It is also the most common in terms of natural occurrence, as it can often be found in soot.
The structure of C60 is a truncated (T = 3) icosahedron, which resembles a soccer ball of the type made of twenty hexagons and twelve pentagons, with a carbon atom at the vertices of each polygon and a bond along each polygon edge.
The van der Waals diameter of a C60 molecule is about 1 nanometer (nm). The nucleus to nucleus diameter of a C60 molecule is about 0.7 nm.
The C60 molecule has two bond lengths. The 6:6 ring bonds (between two hexagons) can be considered "double bonds" and are shorter than the 6:5 bonds (between a hexagon and a pentagon).
Boron buckyball
A new type of buckyball utilizing boron atoms instead of the usual carbon has been predicted and described by researchers at Rice University. The B-80 structure is predicted to be more stable than the C-60 buckyball. [1] One reason for this given by the researchers is that the B-80 is actually more like the original geodesic dome structure popularized by Buckminster Fuller which utilizes triangles rather than hexagons.
Variations of buckballs
Another fairly common buckminsterfullerene is C70,[2] but fullerenes with 72 and even up to 100 carbon atoms are commonly obtained.
In mathematical terms, the structure of a fullerene is a trivalent convex polyhedron with pentagonal and hexagonal faces. In graph theory, the term fullerene refers to any 3-regular, planar graph with all faces of size 5 or 6 (including the external face). It follows from Euler's polyhedron formula, |V|-|E|+|F| = 2, (where |V|, |E|, |F| indicate the number of vertices, edges, and faces), that there are exactly 12 pentagons in a fullerene and |V|/2-10 hexagons.
The smallest fullerene is the dodecahedron—the unique C20, dodecahedrane. There are no fullerenes with 22 vertices. The number of fullerenes C2n grows with increasing n = 12,13,14..., roughly in proportion to n9. For instance, there are 1812 non-isomorphic fullerenes C60. Note that only one form of C60, the buckminsterfullerene alias truncated icosahedron, has no pair of adjacent pentagons (the smallest such fullerene). To further illustrate the growth, there are 214,127,713 non-isomorphic fullerenes C200, 15,655,672 of which have no adjacent pentagons.
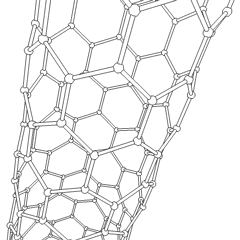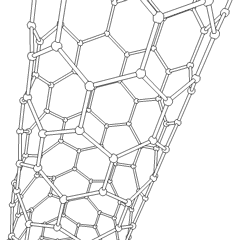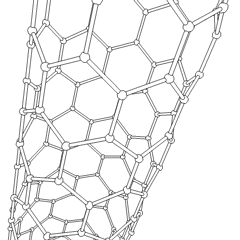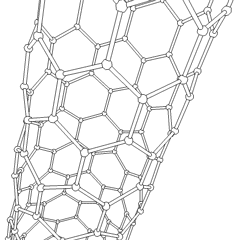
Buckytubes
Carbon nanotubes
Nanotubes are cylindrical fullerenes. These tubes of carbon are usually only a few nanometres wide, but they can range from less than a micrometer to several millimeters in length. They often have closed ends, but can be open-ended as well. There are also cases in which the tube reduces in diameter before closing off. Their unique molecular structure results in extraordinary macroscopic properties, including high tensile strength, high electrical conductivity, high ductility, high resistance to heat, and relative chemical inactivity (as it is cylindrical and 'planar'—that is, it has no 'exposed' atoms that can be easily displaced). One proposed use of carbon nanotubes is in paper batteries , developed in 2007 by researchers at Rensselaer Polytechnic Institute.
Carbon nanobuds
Nanobuds have been obtained by adding Buckminsterfullerenes to carbon nanotubes.
Properties
For the past decade, the chemical and physical properties of fullerenes have been a hot topic in the field of research and development, and are likely to continue to be for a long time. Popular Science has published articles about the possible uses of fullerenes in armor.[citation needed] In April 2003, fullerenes were under study for potential medicinal use: binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma. The October 2005 issue of Chemistry and Biology contains an article describing the use of fullerenes as light-activated antimicrobial agents.[3]
In the field of nanotechnology, heat resistance and superconductivity are some of the more heavily studied properties.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
There are many calculations that have been done using ab-initio Quantum Methods applied to fullerenes. By DFT and TDDFT methods one can obtain IR, Raman and UV spectra. Results of such calculations can be compared with experimental results.
Aromaticity
Researchers have been able to increase the reactivity of fullerenes by attaching active groups to their surfaces. Buckminsterfullerene does not exhibit "superaromaticity": that is, the electrons in the hexagonal rings do not delocalize over the whole molecule.
A spherical fullerene of n carbon atoms has n pi-bonding electrons. These should try to delocalize over the whole molecule. The quantum mechanics of such an arrangement should be like one shell only of the well-known quantum mechanical structure of a single atom, with a stable filled shell for n = 2, 8, 18, 32, 50, 72, 98, 128, etc.; i.e. twice a perfect square; but this series does not include 60. As a result, C60 in water tends to pick up two more electrons and become an anion. The nC60 described below may be the result of C60's trying to form a loose metallic bonding.
Chemistry
Fullerenes are stable, but not totally nonreactive. The sp2-hybridized carbon atoms, which are at their energy minimum in planar graphite, must be bent to form the closed sphere or tube, which produces angle strain. The characteristic reaction of fullerenes is electrophilic addition at 6,6-double bonds, which reduces angle strain by changing sp2-hybridized carbons into sp3-hybridized ones. The change in hybridized orbitals causes the bond angles to decrease from about 120 degrees in the sp2 orbitals to about 109.5 degrees in the sp3 orbitals. This decrease in bond angles allows for the bonds to bend less when closing the sphere or tube, and thus, the molecule becomes more stable.
Other atoms can be trapped inside fullerenes to form inclusion compounds known as endohedral fullerenes. An unusual example is the egg shaped fullerene Tb3N@C84, which violates the isolated pentagon rule.[4] Recent evidence for a meteor impact at the end of the Permian period was found by analysing noble gases so preserved.[5] Metallofullerene-based inoculates using the rhonditic steel process are beginning production as one of the first commercially-viable uses of buckyballs.
Solubility
Fullerenes are sparingly soluble in many solvents. Common solvents for the fullerenes include aromatics, such as toluene, and others like carbon disulfide. Solutions of pure Buckminsterfullerene have a deep purple color. Solutions of C70 are a reddish brown. The higher fullerenes C76 to C84 have a variety of colors. C76 has two optical forms, while other higher fullerenes have several structural isomers. Fullerenes are the only known allotrope of carbon that can be dissolved in common solvents at room temperature.
Some fullerene structures are not soluble because they have a small bandgap between the ground and excited states. These include the small fullerenes C28[6], C36 and C50. The C72 structure is also in this class, but the endohedral version with a trapped lanthanide-group atom is soluble due to the interaction of the metal atom and the electronic states of the fullerene. Researchers had originally been puzzled by C72 being absent in fullerene plasma-generated soot extract, but found in endohedral samples. Small band gap fullerenes are highly reactive and bind to other fullerenes or to soot particles.
Solvents that are able to dissolve buckminsterfullerene (C60) are listed below in order from highest solubility. The value in parentheses is the approximate saturated concentration.[7]
- 1-chloronaphthalene (51 mg/mL)
- 1-methylnaphthalene (33 mg/mL)
- 1,2-dichlorobenzene (24 mg/mL)
- 1,2,4-trimethylbenzene (18 mg/mL)
- tetrahydronaphthalene (16 mg/mL)
- carbon disulfide (8 mg/mL)
- 1,2,3-tribromopropane (8 mg/mL)
- bromoform (5 mg/mL)
- toluene (3 mg/ml)
- benzene (1.5 mg/ml)
- cyclohexane (1.2 mg/ml)
- carbon tetrachloride (0.4 mg/ml)
- chloroform (0.25 mg/ml)
- n-hexane (0.046 mg/ml)
- tetrahydrofuran (0.006 mg/ml)
- acetonitrile (0.004 mg/ml)
- methanol (0.00004 mg/ml)
- water (1.3x10-11 mg/mL)
Solubility of C60 in some solvents shows unusual behavior due to existance of solvate phases (analogues of crystallohydrates). For example, solubility of C60 in beznene solution shows maximum at about 313K. Crystallization from benzene solution at temperatures below maximum results in formation of triclinic solid solvate with four benzene molecules C60*4C6H6 which is rather unstable on air. Out of solution this structure decomposes into usual fcc C60 in few minutes time. At temperatures above solubility maximum the solvate is not stable even when immersed in saturated solution and melts with formation of fcc C60. Crystallization at temperatures above the solubility maximum results in formation of pure fcc C60. Large millimeter size crystals of C60 and C70 can be grown from solution both for solvates and for pure fullerenes.
[8]
[9]
Quantum mechanics
In 1999, researchers from the University of Vienna demonstrated that the wave-particle duality applied to molecules such as fullerene[10]. One of the co-authors of this research, Julian Voss-Andreae became an artist and has since created several sculptures symbolizing wave-particle duality in Buckminsterfullerenes.
Science writer Marcus Chown made a reference on the CBC radio show "Quirks And Quarks" in May 2006 that there is a scientist working on having buckyballs follow the quantum behavior of atoms of appearing to be in two places at once. The work is continuing on this phenomenon.
Safety
Mori T et al. 2006. Toxicology, 225; pp. 48-54. studied in vitro genotoxicity and mutagenicity, and LD50 values in rodents for C60 and C70 mixtures. No evidence was found of any genotoxic or mutagenic potential and the rats tolerated 2g/kg oral dosing with no adverse effects.
In addition, many other studies have shown fullerenes to be non-toxic. A comprehensive and recent review of work on fullerene toxicity is available in "Toxicity Studies of Fullerenes and Derivatives," a chapter from the book "Bio-applications of Nanoparticles" (Chan ed., Landes Bioscience, 2007). In this work, the authors review the work on fullerene toxicity beginning in the early 1990's to present, and conclude that the evidence gathered since the discovery of fullerenes overwhelmingly points to C60 being non-toxic.
Popular culture
Examples of fullerenes in popular culture are numerous. In fact, fullerenes appeared in fiction well before science started to take serious interest in them.
- It is the topic of a science fiction book named Decipher written by Stel Pavlou
- In New Scientist there used to be a weekly column called Daedalus written by David Jones, which contained humorous descriptions of unlikely technologies. In 1966 the columnist included a description of C60 and other forms of graphite. This was meant as pure entertainment.
- Also in New Scientist magazine, a free book was enclosed entitled, "100 Things to Do Before You Die," one of which was to kick a buckyball.
- The buckyball is the state molecule of Texas [11]
See also
Notes
- ↑ Boyd, Jade. 2007. Bucky's brother—The boron buckyball makes its debut. eurekalert.org. Retrieved December 4, 2007.
- ↑ Buckminsterfullerene, C60. University of Bristol. Retrieved December 4, 2007.
- ↑ Tegos, G., T. Demidova, D. Arcila-Lopez, H. Lee, T. Wharton, H. Gali, M. Hamblin. 2005. Cationic Fullerenes Are Effective and Selective Antimicrobial Photosensitizers. Chemistry & Biology. 12:10:1127-1135. Retrieved December 4, 2007.
- ↑ Beavers, Christine M., Tianming Zuo, James C. Duchamp, Kim Harich, Harry C. Dorn, Marilyn M. Olmstead, and Alan L. Balch. 2006. Tb3N@C84: An Improbable, Egg-Shaped Endohedral Fullerene that Violates the Isolated Pentagon Rule. J. Am. Chem. Soc. 128:35:11352-11353. Retrieved December 4, 2007.
- ↑ Becker, Luann, Robert J. Poreda, Andrew G. Hunt, Theodore E. Bunch, Michael Rampino. 2007. Impact Event at the Permian-Triassic Boundary: Evidence from Extraterrestrial Noble Gases in Fullerenes. Science. 291:5508:1530-3. Retrieved December 4, 2007.
- ↑ Guo, Ting, Richard E. Smalley, Gustavo E. Scuseria. 1993. Ab initio theoretical predictions of C28, C28H4, C28F4, (Ti at C28)H4, and M at C28 (M = Mg, Al, Si, S, Ca, Sc, Ti, Ge, Zr, and Sn). Journal of Chemical Physics. 99:1:352-359. Retrieved December 4, 2007.
- ↑ Bezmel'nitsyn, V.N., A.V. Eletskiĭ, M.V. Okun'. 1998. Fullerenes in Solutions. Uspekhi Fizicheskikh Nauk, Russian Academy of Sciences, 41.
- ↑ Talyzin A.V. 1997. Phase transition C60-C60*4C6H6 in liquid benzene. J. of Phys.Chem. 101:47.
- ↑ Talyzin A.V., I. Engstrцm. 1998. C70 in a Benzene, Hexane and Toluene solutions. J. of Phys.Chem. 102:34:6477-6481.
- ↑ Arndt, M., O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, Anton Zeilinger. 1999. Wave-particle duality of C60. Nature. 401:680-682.
- ↑ State molecule of Texas. Texas Legislature Online. Retrieved December 4, 2007.
ReferencesISBN links support NWE through referral fees
- Aldersey-Williams, Hugh. 1995. The Most Beautiful Molecule: The Discovery of the Buckyball. John Wiley. ISBN 0-471-19333-X.
- Dresselhaus, M. S., G. Dresselhaus, and P. C. Eklund. 1996. Science of Fullerenes and Carbon Nanotubes. San Diego: Academic Press. ISBN 0122218205.
- Hirsch, Andreas, and Michael Brettreich. 2005. Fullerenes: Chemistry and Reactions. Weinheim: Wiley-VCH. ISBN 3527308202.
- Lakhtakia, A. 2004. The Handbook of Nanotechnology: Nanometer Structure Theory, Modelling, and Simulation. Bellingham, WA: SPIE. ISBN 081945186X.
- Margadonna, Serena. 2007. Fullerene-Related Materials. Dordrecht: Springer. ISBN 978-1402044588.
- Nalwa, Hari Singh. 2004. Encyclopedia of Nanoscience and Nanotechnology. Stevenson Ranch, CA: American Scientific Publishers. ISBN 1588830012.
External links
- Fullerenes in solution: about anomalous temperature dependence of solubility and solid solvates of fullerenes
- Fullerene and nanotube Gallery
- Properties of C60 fullerene
- [1]
- Buckyball Workshops by Sir Harry Kroto and the Vega Science Trust
- Center for Nanoscale Science and Technology
- Center for Biological and Environmental Nanotechnology
- Dr. Smalley's brief autobiography
- Dr. Smalley's webpage
- Sir Harry Kroto's webpage
- Interview with James R. Heath, discussing the discovery of C60
- Carbon Fullerene & Nanotube Models Vincent Herr, Houston, TX
- Diffraction and Interference with Fullerenes: Wave-particle duality of C60, University of Vienna
- Fullerene-based architectures for quantum computing in Germany and in Great Britain at the QIP IRC
- Molview from bluerhinos.co.uk See Buckminsterfullerene (C60) in 3D
- Computational Chemistry Wiki
- A Spherical Revelation
- C60 3D-view and pdb-file
- Simple model of Fullerene.
- Story on "Buckyeggs" (UC Davis website)
- Stainless Steel Buckminster Fullerenes
- Rhonditic Steel
| |||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.