Difference between revisions of "Buffer solution" - New World Encyclopedia
| Line 23: | Line 23: | ||
When writing about buffer systems they can be represented as salt of conjugate base/acid, or base/salt of conjugate acid. | When writing about buffer systems they can be represented as salt of conjugate base/acid, or base/salt of conjugate acid. | ||
| − | ==Calculating pH== | + | ===Calculating pH=== |
| − | The equilibrium above has the following [[acid dissociation constant]]: | + | The equilibrium reaction above has the following [[acid dissociation constant]]: |
:<math> \mathrm{K_a = \frac{[H^+][A^-]}{[HA]}}</math> | :<math> \mathrm{K_a = \frac{[H^+][A^-]}{[HA]}}</math> | ||
Revision as of 20:05, 29 November 2007
- Acid-base extraction
- Acid-base reaction
- Acid dissociation constant
- Acidity function
- Buffer solutions
- pH
- Proton affinity
- Self-ionization of water
- Acids:
- Lewis acids
- Mineral acids
- Organic acids
- Strong acids
- Superacids
- Weak acids
- Bases:
- Lewis bases
- Organic bases
- Strong bases
- Superbases
- Non-nucleophilic bases
- Weak bases
Buffer solutions are solutions that resist changes in pH (by resisting changes in hydronium ion and hydroxide ion concentrations) upon addition of small amounts of acid or base, or upon dilution. Buffer solutions usually consist of a weak acid and its conjugate base, or, less commonly, a weak base and its conjugate acid.
Buffer solutions are used in industry for chemical manufacturing and fermentation processes, and to set the proper conditions for dyeing fabrics. In research laboratories, buffers are used for chemical analyses, syntheses, and calibration of pH meters. In living organisms, buffer solutions maintain the correct pH for many enzymes to work. Blood plasma contains a buffer (of carbonic acid and bicarbonate) to maintain a pH of approximately 7.4.
This article discusses buffer solutions prepared with water, but not other solvents. Also, these solutions are presented in terms of the Brønsted-Lowry notion of acids and bases, not the Lewis acid-base theory.
How buffers work
The ability of a buffer solution to resist changes in pH is the result of the equilibrium between a weak acid (HA) and its conjugate base (A−):
- HA(aq) + H2O(l) → H3O+(aq) + A−(aq)
Any alkali added to the solution is consumed by the Hydronium ions. These ions are mostly regenerated as the equilibrium moves to the right and some of the acid dissociates into Hydronium ions and the conjugate base. If a strong acid is added, the conjugate base is protonated, and the pH is almost entirely restored. This is an example of Le Chatelier's principle and the common ion effect.
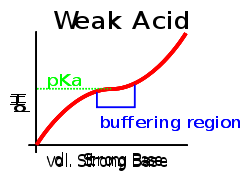
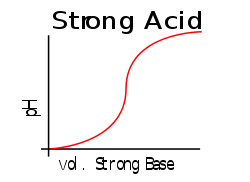
This contrasts with solutions of strong acids or strong bases, where any additional strong acid or base can greatly change the pH. This may be easier to see by comparing two graphs when an strong acid is titrated with a strong base the curve will have a large gradient throughout showing that a small addition of base/acid will have a large effect compared to a weak acid/strong base titration curve which will have a smaller gradient near the pKa.
When writing about buffer systems they can be represented as salt of conjugate base/acid, or base/salt of conjugate acid.
Calculating pH
The equilibrium reaction above has the following acid dissociation constant:
Simple manipulation with logarithms gives the Henderson-Hasselbalch equation, which describe pH in terms of pKa:
In this equation
- [A−] is the concentration of the conjugate base. This may be considered as coming completely from the salt, since the acid supplies relatively few anions compared to the salt.
- [HA] is the concentration of the acid. This may be considered as coming completely from the acid, since the salt supplies relatively few complete acid molecules (A − may extract H + from water to become HA) compared to the added acid.
Maximum buffering capacity is found when pH = pKa, and buffer range is considered to be at a pH = pKa ± 1.
Illustration of buffering effect: Sodium acetate/acetic acid
The acid dissociation constant for acetic acid-sodium acetate is given by the equation:
Since this equilibrium only involves a weak acid and base, it can be assumed that ionization of the acetic acid and hydrolysis of the acetate ions are negligible. In a buffer consisting of equal amounts of acetic acid and sodium acetate, the equilibrium equation simplifies to
- ,
and the pH of the buffer as is equal to the pKa.
To determine the effect of addition of a strong acid such as HCl, the following mathematics would provide the new pH. Since HCl is a strong acid, it is completely ionized in solution. This increases the concentration of H+ in solution, which then neutralizes the acetate by the following equation.
The consumed hydrogen ions change the effective number of moles of acetic acid and acetate ions:
After accounting for volume change to determine concentrations, the new pH could be calculated from the Henderson-Hasselbalch equation. Any neutralization will result in a small change in pH, since it is on a logarithmic scale..
Applications
Their resistance to changes in pH makes buffer solutions very useful for chemical manufacturing and essential for many biochemical processes. The ideal buffer for a particular pH has a pKa equal to the pH desired, since a solution of this buffer would contain equal amounts of acid and base and be in the middle of the range of buffering capacity.
Buffer solutions are necessary to keep the correct pH for enzymes in many organisms to work. Many enzymes work only under very precise conditions; if the pH strays too far out of the margin, the enzymes slow or stop working and can denature, thus permanently disabling its catalytic activity. A buffer of carbonic acid (H2CO3) and bicarbonate (HCO3−) is present in blood plasma, to maintain a pH between 7.35 and 7.45.
Industrially, buffer solutions are used in fermentation processes and in setting the correct conditions for dyes used in colouring fabrics. They are also used in chemical analysis and calibration of pH meters.
Common buffer compounds used in biology
| Common Name | pKa at 25°C |
Buffer Range | Temp Effect (pH / °C)** |
Mol. Weight |
Full Compound Name |
|---|---|---|---|---|---|
| TAPS | 8.43 | 7.7 – 9.1 | −0.018 | 243.3 | 3-{[tris(hydroxymethyl)methyl]amino}propanesulfonic acid |
| Bicine | 8.35 | 7.6 – 9.0 | −0.018 | 163.2 | N,N-bis(2-hydroxyethyl)glycine |
| Tris | 8.06 | 7.5 – 9.0 | −0.028 | 121.14 | tris(hydroxymethyl)methylamine |
| Tricine | 8.05 | 7.4 – 8.8 | −0.021 | 179.2 | N-tris(hydroxymethyl)methylglycine |
| HEPES | 7.48 | 6.8 – 8.2 | −0.014 | 238.3 | 4-2-hydroxyethyl-1-piperazineethanesulfonic acid |
| TES | 7.40 | 6.8 – 8.2 | −0.020 | 229.20 | 2-{[tris(hydroxymethyl)methyl]amino}ethanesulfonic acid |
| MOPS | 7.20 | 6.5 – 7.9 | −0.015 | 209.3 | 3-(N-morpholino)propanesulfonic acid |
| PIPES | 6.76 | 6.1 – 7.5 | −0.008 | 302.4 | piperazine-N,N′-bis(2-ethanesulfonic acid) |
| Cacodylate | 6.27 | 5.0 – 7.4 | 138.0 | dimethylarsinic acid | |
| MES | 6.15 | 6.1 – 7.5 | −0.011 | 195.2 | 2-(N-morpholino)ethanesulfonic acid |
| Acetate | 4.76 | 3.8 – 5.8 | 59.04 | — |
** Values are approximate
Making buffer solutions
In general, preparing a buffer solution requires either:
- A weak acid and a salt of the acid's conjugate base
- Or a weak base and a salt of the base's conjugate acid
Both of which in sufficient amounts to maintain the ability to buffer
Example: Citric acid-phosphate buffer
Make up 0.1M citric acid and 0.2M Disodium hydrogen phosphate solutions then mix as follows to make a 100 ml solution:
| pH | 0.2M Na2HPO4 | 0.1M Citric Acid |
|---|---|---|
| 3.0 | 20.55 ml | 79.45 ml |
| 4.0 | 38.55 ml | 61.45 ml |
| 5.0 | 51.50 ml | 48.50 ml |
| 6.0 | 63.15 ml | 36.85 ml |
| 7.0 | 82.35 ml | 17.65 ml |
| 8.0 | 97.25 ml | 2.75 ml |
Buffering agent
A buffering agent adjusts the pH of a solution. The function of a buffering agent is to drive an acidic or alkaline solution to a certain pH state and prevent a change in this pH. Buffering agents have variable properties — some are more soluble than others; some are acidic while others are basic. As pH managers, they are important in many chemical applications, including agriculture, food processing, medicine and photography.
What a buffering agent is
Buffering agents can be either the weak acid or weak base that would comprise a buffer solution. Buffering agents are usually added to water to form buffer solutions. They are the substances that are responsible for the buffering seen in these solutions. These agents are added to substances that are to be placed into acidic or basic conditions in order to stabilize the substance. For example, buffered aspirin has a buffering agent, such as MgO, that will maintain the pH of the aspirin as it passes through the stomach of the patient. Another use of a buffering agent is in antacid tablets, whose primary purpose is to lower the acidity of the stomach.
How a buffering agent works
The way buffering agents work is seen in how buffer solutions work. Using Le Chatelier's principle we get an equilibrium expression between the acid and conjugate base. As a result we see that there is little change in the concentrations of the acid and base so therefore the solution is buffered. A buffering agent sets up this concentration ratio by providing the corresponding conjugate acid or base to stabilize the pH of that which it is added to. The resulting pH of this combination can be found by using the Henderson-Hasselbalch equation:
where HA is the weak acid and A is the anion of the base.
Buffering Agents Vs. Buffer Solutions
Buffering agents are similar to buffer solutions as a result of the fact that buffering agents are the main components of a buffer solution. They both regulate the pH of a solution and resist changes in pH. A buffer solution maintains the pH for the whole system which is placed into it, whereas a buffering agent is added to an already acidic or basic solution, which it then modifies and maintains a new pH.
Buffering agents and buffer solutions are almost one and the same except for a few differences:
- Solutions maintain pH of a system, preventing large changes in it, whereas agents modify the pH of what they are placed into
- Agents are the active components of a buffer solutions.
Examples
Monopotassium phosphate (MKP) is an example of a buffering agent. It has a mildly acidic reaction; when applied as a fertilizer with urea or diammonium phosphate, it minimizes pH fluctuations which can cause nitrogen loss.
See also
- Acid
- Base (chemistry)
- Common-ion effect
- Links to external chemical sources
ReferencesISBN links support NWE through referral fees
- Atkins, Peter, and Loretta Jones. 2008. Chemical Principles: The Quest for Insight. 4th ed. New York: W.H. Freeman. ISBN 978-0716799030.
- Chang, Raymond. 2006. Chemistry. 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031.
- Cotton, F. Albert, and Geoffrey Wilkinson. 1980. Advanced Inorganic Chemistry. 4th ed. New York: Wiley. ISBN 0471027758.
- Harris, Daniel C. 2006. Quantitative Chemical Analysis. 7th ed. New York: W. H. Freeman. ISBN 978-0716776949.
- McMurry, J., and R.C. Fay. 2004. Chemistry. 4th ed. Upper Saddle River, NJ: Prentice Hall. ISBN 0131402080.
External links
- Derivation and discussion of Henderson-Hasselbalch equation. Retrieved November 17, 2007.
- Glossary. Ōshun Supply. Retrieved November 17, 2007.
- General Chemistry FAQ: buffered aspirin. Retrieved November 17, 2007.
- Effects of pH on Pesticides and Growth Regulators. Retrieved November 17, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
![{\displaystyle \mathrm {K_{a}={\frac {[H^{+}][A^{-}]}{[HA]}}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/14a41818964da5798042c1d67a87ead832bce748)
![{\displaystyle pH=pK_{a}+log_{10}{\frac {[A^{-}]}{[HA]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e4f6fb8355d64468056c75106ed979a6122d5b2b)
![{\displaystyle \mathrm {K_{a}={\frac {[H^{+}][CH_{3}COO^{-}]}{[CH_{3}COOH]}}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/e0241dab101bee7364ef4f8565e01e4a1cfa1d79)
![{\displaystyle \mathrm {K_{a}=[H^{+}]} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/203cc492d8e93bcc8e576b29dd52a365ecc976f1)



![{\displaystyle {\mbox{pH}}={\mbox{pKa}}+\log _{10}{\frac {\left[{\mbox{A}}^{-}\right]}{\left[{\mbox{HA}}\right]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ecc78f9b46f86bb705e52815287932b870b04c2c)