Difference between revisions of "Addiction" - New World Encyclopedia
| Line 265: | Line 265: | ||
The general classes of epigenetic alterations that have been implicated in transgenerational epigenetic inheritance include [[DNA methylation]], [[histone modification]]s, and [[Downregulation and upregulation|downregulation or upregulation]] of [[microRNA]]s.<ref name="Transgenerational epigenetic inheritance in addiction" /> With respect to addiction, more research is needed to determine the specific [[Heredity|heritable]] epigenetic alterations that arise from various forms of addiction in humans and the corresponding behavioral phenotypes from these epigenetic alterations that occur in human offspring.<ref name="Transgenerational epigenetic inheritance in addiction" /><ref name="pmid26572641" /> Based upon preclinical evidence from [[animal research]], certain addiction-induced epigenetic alterations in rats can be transmitted from parent to offspring and produce behavioral phenotypes that decrease the offspring's risk of developing an addiction.{{#tag:ref|According to a review of experimental animal models that examined the transgenerational epigenetic inheritance of [[epigenetic mark]]s that occur in addiction, alterations in [[histone acetylation]] – specifically, di-acetylation of [[lysine]] [[residue (amino acid)|residues]] 9 and 14 on [[histone 3]] (i.e., [[H3K9ac2]] and [[H3K14ac2]]) in association with [[BDNF]] [[gene promoter]]s – have been shown to occur within the [[medial prefrontal cortex]] (mPFC), [[testes]], and [[sperm]] of cocaine-addicted male rats.<ref name="Transgenerational epigenetic inheritance in addiction" /> These epigenetic alterations in the rat mPFC result in increased BDNF [[gene expression]] within the mPFC, which in turn blunts the [[reward system|rewarding properties]] of cocaine and reduces cocaine [[self-administration]].<ref name="Transgenerational epigenetic inheritance in addiction" /> The male but not female offspring of these cocaine-exposed rats inherited both epigenetic marks (i.e., di-acetylation of lysine residues 9 and 14 on histone 3) within mPFC neurons, the corresponding increase in BDNF expression within mPFC neurons, and the behavioral phenotype associated with these effects (i.e., a reduction in cocaine reward, resulting in reduced cocaine-seeking by these male offspring).<ref name="Transgenerational epigenetic inheritance in addiction" /> Consequently, the transmission of these two cocaine-induced epigenetic alterations (i.e., H3K9ac2 and H3K14ac2) in rats from male fathers to male offspring served to reduce the offspring's risk of developing an addiction to cocaine.<ref name="Transgenerational epigenetic inheritance in addiction" /> {{As of|2018|post=,}} neither the heritability of these epigenetic marks in humans nor the behavioral effects of the marks within human mPFC neurons has been established.<ref name="Transgenerational epigenetic inheritance in addiction" />|group="note"}}<ref name="Transgenerational epigenetic inheritance in addiction" /> More generally, the heritable behavioral phenotypes that are derived from addiction-induced epigenetic alterations and transmitted from parent to offspring may serve to either increase or decrease the offspring's risk of developing an addiction.<ref name="Transgenerational epigenetic inheritance in addiction" /><ref name="pmid26572641" /> | The general classes of epigenetic alterations that have been implicated in transgenerational epigenetic inheritance include [[DNA methylation]], [[histone modification]]s, and [[Downregulation and upregulation|downregulation or upregulation]] of [[microRNA]]s.<ref name="Transgenerational epigenetic inheritance in addiction" /> With respect to addiction, more research is needed to determine the specific [[Heredity|heritable]] epigenetic alterations that arise from various forms of addiction in humans and the corresponding behavioral phenotypes from these epigenetic alterations that occur in human offspring.<ref name="Transgenerational epigenetic inheritance in addiction" /><ref name="pmid26572641" /> Based upon preclinical evidence from [[animal research]], certain addiction-induced epigenetic alterations in rats can be transmitted from parent to offspring and produce behavioral phenotypes that decrease the offspring's risk of developing an addiction.{{#tag:ref|According to a review of experimental animal models that examined the transgenerational epigenetic inheritance of [[epigenetic mark]]s that occur in addiction, alterations in [[histone acetylation]] – specifically, di-acetylation of [[lysine]] [[residue (amino acid)|residues]] 9 and 14 on [[histone 3]] (i.e., [[H3K9ac2]] and [[H3K14ac2]]) in association with [[BDNF]] [[gene promoter]]s – have been shown to occur within the [[medial prefrontal cortex]] (mPFC), [[testes]], and [[sperm]] of cocaine-addicted male rats.<ref name="Transgenerational epigenetic inheritance in addiction" /> These epigenetic alterations in the rat mPFC result in increased BDNF [[gene expression]] within the mPFC, which in turn blunts the [[reward system|rewarding properties]] of cocaine and reduces cocaine [[self-administration]].<ref name="Transgenerational epigenetic inheritance in addiction" /> The male but not female offspring of these cocaine-exposed rats inherited both epigenetic marks (i.e., di-acetylation of lysine residues 9 and 14 on histone 3) within mPFC neurons, the corresponding increase in BDNF expression within mPFC neurons, and the behavioral phenotype associated with these effects (i.e., a reduction in cocaine reward, resulting in reduced cocaine-seeking by these male offspring).<ref name="Transgenerational epigenetic inheritance in addiction" /> Consequently, the transmission of these two cocaine-induced epigenetic alterations (i.e., H3K9ac2 and H3K14ac2) in rats from male fathers to male offspring served to reduce the offspring's risk of developing an addiction to cocaine.<ref name="Transgenerational epigenetic inheritance in addiction" /> {{As of|2018|post=,}} neither the heritability of these epigenetic marks in humans nor the behavioral effects of the marks within human mPFC neurons has been established.<ref name="Transgenerational epigenetic inheritance in addiction" />|group="note"}}<ref name="Transgenerational epigenetic inheritance in addiction" /> More generally, the heritable behavioral phenotypes that are derived from addiction-induced epigenetic alterations and transmitted from parent to offspring may serve to either increase or decrease the offspring's risk of developing an addiction.<ref name="Transgenerational epigenetic inheritance in addiction" /><ref name="pmid26572641" /> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Epidemiology== | ==Epidemiology== | ||
Revision as of 17:09, 28 May 2020
Currently working on —Jennifer Tanabe (talk) May 2020
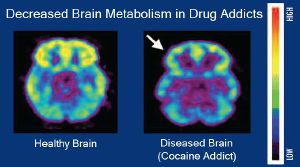
Addiction is a brain disorder characterized by compulsive engagement in rewarding stimuli despite adverse consequences.[7] Despite the involvement of a number of psychosocial factors, a biological process—one that is induced by repeated exposure to an addictive stimulus—is the core pathology that drives the development and maintenance of an addiction.[1][8] The two properties that characterize all addictive stimuli are that they are reinforcing (i.e., they increase the likelihood that a person will seek repeated exposure to them) and intrinsically rewarding (i.e., they are perceived as being inherently positive, desirable, and pleasurable).[1][2][6]
Classic hallmarks of addiction include impaired control over substances or behavior, preoccupation with substance or behavior, and continued use despite consequences.[9] Habits and patterns associated with addiction are typically characterized by immediate gratification (short-term reward), coupled with delayed deleterious effects (long-term costs).[10]
Types of addiction
| Addiction and dependence glossary | |
* addiction – a biopsychosocial disorder characterized by compulsively seeking to achieve a desired effect, such as intoxication, despite harm and adverse consequences to self and others
|
Addiction canonically refers to substance abuse; however, the term connotation has been expanded to include behaviors that may lead to a reward (e.g., gambling, eating, or shopping)[11] since the 1990s. A gene transcription factor known as ΔFosB has been identified as a necessary common factor involved in both behavioral and drug addictions, which are associated with the same set of neural adaptations in the reward system.[12][13][14]
Examples of drug and behavioral addictions include alcoholism, marijuana addiction, amphetamine addiction, cocaine addiction, nicotine addiction, opioid addiction, food addiction, video game addiction, gambling addiction, and sexual addiction. The only behavioral addiction recognized by the DSM-5 and the ICD-10 is gambling addiction. With the introduction of the ICD-11 gaming addiction was appended.[15] The term addiction is misused frequently to refer to other compulsive behaviors or disorders, particularly dependence, in news media.[16] An important distinction between drug addiction and dependence is that drug dependence is a disorder in which cessation of drug use results in an unpleasant state of withdrawal, which can lead to further drug use.[17] Addiction is the compulsive use of a substance or performance of a behavior that is independent of withdrawal. Addiction can occur in the absence of dependence, and dependence can occur in the absence of addiction, although the two often occur together.
Addiction exacts an "astoundingly high financial and human toll" on individuals and society as a whole.[18][19][20] In the United States, the total economic cost to society is greater than that of all types of diabetes and all cancers combined.[20] These costs arise from the direct adverse effects of drugs and associated healthcare costs (e.g., emergency medical services and outpatient and inpatient care), long-term complications (e.g., lung cancer from smoking tobacco products, liver cirrhosis and dementia from chronic alcohol consumption, and meth mouth from methamphetamine use), the loss of productivity and associated welfare costs, fatal and non-fatal accidents (e.g., traffic collisions), suicides, homicides, and incarceration, among others.[18][19][20][21]
Biomolecular mechanisms
ΔFosB, a gene transcription factor, has been identified as playing a critical role in the development of addictive states in both behavioral addictions and drug addictions.[12][13][14] Overexpression of ΔFosB in the nucleus accumbens is necessary and sufficient for many of the neural adaptations seen in drug addiction;[12] it has been implicated in addictions to alcohol, cannabinoids, cocaine, nicotine, phenylcyclidine, and substituted amphetamines[12][22][23][24] as well as addictions to natural rewards such as sex, exercise, and food.[13][14] A recent study also demonstrated a cross-sensitization between drug reward (amphetamine) and a natural reward (sex) that was mediated by ΔFosB.[25]
Besides increased ΔFosB expression in the nucleus accumbens, there are many other correlations in the neurobiology of behavioral addictions with drug addictions.
Behaviors like gambling have been linked to the new found idea of the brain's capacity to anticipate rewards. The reward system can be triggered by early detectors of the behavior, and trigger dopamine neurons to begin stimulating behaviors. But in some cases, it can lead to many issues due to error, or reward-prediction errors. These errors can act as teaching signals to create a complex behavior task over time.[26]
One of the most important discoveries of addictions has been the drug based reinforcement and, even more important, reward based learning processes. Several structures of the brain are important in the conditioning process of behavioral addiction; these subcortical structures form the brain regions known as the reward system. One of the major areas of study is the amygdala, a brain structure which involves emotional significance and associated learning. Research shows that dopaminergic projections from the ventral tegmental area facilitate a motivational or learned association to a specific behavior.[27] Dopamine neurons take a role in the learning and sustaining of many acquired behaviors. Research specific to Parkinson's disease has led to identifying the intracellular signaling pathways that underlie the immediate actions of dopamine. The most common mechanism of dopamine is to create addictive properties along with certain behaviors.[28] There are three stages to the dopamine reward system: bursts of dopamine, triggering of behavior, and further impact to the behavior. Once electronically signaled, possibly through the behavior, dopamine neurons let out a ‘burst-fire’ of elements to stimulate areas along fast transmitting pathways. The behavior response then perpetuates the striated neurons to further send stimuli. The fast firing of dopamine neurons can be monitored over time by evaluating the amount of extracellular concentrations of dopamine through micro dialysis and brain imaging. This monitoring can lead to a model in which one can see the multiplicity of triggering over a period of time.[26] Once the behavior is triggered, it is hard to work away from the dopamine reward system.
Behavioral addiction
Behavioral addiction[note 1] is a form of addiction that involves a compulsion to engage in a rewarding non-substance-related behavior – sometimes called a natural reward[12][13] – despite any negative consequences to the person's physical, mental, social or financial well-being.[32] An addictive behavior is a behavior, or a stimulus related to a behavior (e.g., sex or food), that is both rewarding and reinforcing, and is associated with the development of an addiction. Addictions involving addictive behaviors are normally referred to as behavioral addictions.
The term behavioral addiction refers to a compulsion to engage in a natural reward – which is a behavior that is inherently rewarding (i.e., desirable or appealing) – despite adverse consequences.[5][13][12] Preclinical evidence has demonstrated that marked increases in the expression of ΔFosB through repetitive and excessive exposure to a natural reward induces the same behavioral effects and neuroplasticity as occurs in a drug addiction.[13][33][34][35]
Reviews of both clinical research in humans and preclinical studies involving ΔFosB have identified compulsive sexual activity – specifically, any form of sexual intercourse – as an addiction (i.e., sexual addiction).[13][33] Moreover, reward cross-sensitization between amphetamine and sexual activity, meaning that exposure to one increases the desire for both, has been shown to occur preclinically and clinically as a dopamine dysregulation syndrome;[13][33][34][35] ΔFosB expression is required for this cross-sensitization effect, which intensifies with the level of ΔFosB expression.[13][34][35]
Reviews of preclinical studies indicate that long-term frequent and excessive consumption of high fat or sugar foods can produce an addiction (food addiction).[13][12]
Gambling provides a natural reward which is associated with compulsive behavior and for which clinical diagnostic manuals, namely the DSM-5, have identified diagnostic criteria for an "addiction".[13] In order for a person's gambling behavior to meet criteria of an addiction, it shows certain characteristics, such as mood modification, compulsivity, and withdrawal. There is evidence from functional neuroimaging that gambling activates the reward system and the mesolimbic pathway in particular.[13][36] Similarly, shopping and playing video games are associated with compulsive behaviors in humans and have also been shown to activate the mesolimbic pathway and other parts of the reward system.[13] Based upon this evidence, gambling addiction, video game addiction, and shopping addiction are classified accordingly.[13][36]
Psychiatric and medical classifications
Diagnostic models do not currently include the criteria necessary to identify behaviors as addictions in a clinical setting. Behavioral addictions have been proposed as a new class in DSM-5, but the only category included is gambling addiction. Internet gaming addiction is included in the appendix as a condition for further study.[37][38]
Behavioral addictions, which are sometimes referred to as impulse control disorders, are increasingly recognized as treatable forms of addiction.[39] The type of excessive behaviors identified as being addictive include gambling, food, chocolate, sexual intercourse, use of pornography, use of computers, playing video games, use of the internet and other digital media, exercise, and shopping.
Researching addiction to food, for example, a 2009 Scripps Research Institute study found evidence that the same molecular mechanisms correlated with human drug addiction also exist in compulsive overeating in obese rats. The dopamine D2 receptor studied is associated with vulnerability to drug addiction in humans. It was found downregulated in obese rats exposed to a high fat diet, and further reductions of the receptor increased compulsive eating. The D2 receptor responds to dopamine, a central neurotransmitter released in anticipation of rewarding, satiating experiences such as those involving food, sex or psychoactive drugs.[40]
In August 2011, the American Society of Addiction Medicine (ASAM) issued a public statement defining all addiction in terms of brain changes. "Addiction is a primary, chronic disease of brain reward, motivation, memory and related circuitry."[41]
The following excerpts are taken from the organization's FAQs:
The new ASAM definition makes a departure from equating addiction with just substance dependence, by describing how addiction is also related to behaviors that are rewarding. This is the first time that ASAM has taken an official position that addiction is not solely "substance dependence." This definition says that addiction is about functioning and brain circuitry and how the structure and function of the brains of persons with addiction differ from the structure and function of the brains of persons who do not have addiction. It talks about reward circuitry in the brain and related circuitry, but the emphasis is not on the external rewards that act on the reward system. Food and sexual behaviors and gambling behaviors can be associated with the "pathological pursuit of rewards" described in this new definition of addiction.
We all have the brain reward circuitry that makes food and sex rewarding. In fact, this is a survival mechanism. In a healthy brain, these rewards have feedback mechanisms for satiety or 'enough.' In someone with addiction, the circuitry becomes dysfunctional such that the message to the individual becomes ‘more’, which leads to the pathological pursuit of rewards and/or relief through the use of substances and behaviors. So, anyone who has addiction is vulnerable to food and sex addiction.
Since ASAM released its statement, and shortly before its release, additional new studies have come out on Internet addiction. They reveal the same fundamental brain changes seen in other addicts of drugs.[42][43][44][45][46][47] Another 2011 study found that the risk of Internet addiction in men was about three times more than women. Researchers noted,
Internet addiction is a psychosocial disorder and its characteristics are as follows: tolerance, withdrawal symptoms, affective disorders, and problems in social relations. Internet usage creates psychological, social, school and/or work difficulties in a person's life. Eighteen percent of study participants were considered to be pathological Internet users, whose excessive use of the Internet was causing academic, social, and interpersonal problems. Excessive Internet use may create a heightened level of psychological arousal, resulting in little sleep, failure to eat for long periods, and limited physical activity, possibly leading to the user experiencing physical and mental health problems such as depression, OCD, low family relationships and anxiety.[48]
Another growing area is social media addiction. Psychology researchers surveyed 253 undergraduate students at the University of Albany and found that not only is social media (particularly Facebook) itself potentially addictive, those who use it may also be at greater risk for substance abuse.[49]
Treatment
Behavioral addiction is a treatable condition. Treatment options include psychotherapy and psychopharmacotherapy (i.e., medications) or a combination of both. Cognitive behavioral therapy (CBT) is the most common form of psychotherapy used in treating behavioral addictions; it focuses on identifying patterns that trigger compulsive behavior and making lifestyle changes to promote healthier behaviors. Because cognitive behavioral therapy is considered a short term therapy, the number of sessions for treatment normally ranges from five to twenty. During the session, therapists will lead patients through the topics of identifying the issue, becoming aware of one's thoughts surround the issue, identifying any negative or false thinking, and reshaping said negative and false thinking. While CBT does not cure behavioral addiction, it does help with coping with the condition in a healthy way. Currently, there are no medications approved for treatment of behavioral addictions in general, but some medications used for treatment of drug addiction may also be beneficial with specific behavioral addictions.[36] Any unrelated psychiatric disorders should be kept under control, and differentiated from the contributing factors that cause the addiction.
Substance use disorder
Substance use disorder (SUD), also known as a drug use disorder, is the persistent use of drugs (including alcohol) despite substantial harm and adverse consequences.[50][51] Substance use disorders are characterized by an array of mental, physical, and behavioral symptoms that may cause problems related to loss of control, strain to one's interpersonal life, hazardous use, tolerance, and withdrawal.[52] Drug classes that are involved in SUD include: alcohol; caffeine; cannabis; hallucinogens (such as arylcyclohexylamines); other hallucinogens (such as LSD); inhalants; opioids; sedatives, hypnotics, or anxiolytics; stimulants; tobacco; and other or unknown substances.[53]
In the current Diagnostic and Statistical Manual of Mental Disorders, DSM-5, substance abuse and substance dependence have been merged into the category of substance use disorders.[54][55] The severity of substance use disorders can vary widely; in the diagnosis of a SUD, the severity of an individual's SUD is qualified as mild, moderate, or severe on the basis of how many of the 11 diagnostic criteria are met. The International Classification of Diseases 11th revision (ICD-11) divides substance use disorders into two categories: (1) harmful pattern of substance use; and (2) substance dependence.[56]
In 2017 globally 271 million people (5.5% of adults) were estimated to have used one or more illicit drugs.[57] Of these 35 million had a substance use disorder.[57] An additional 237 million men and 46 million women have alcohol use disorder as of 2016.[58] In 2017 substance use disorders from illicit substances directly resulted in 585,000 deaths.[57] Direct deaths from drug use, other than alcohol, have increased over 60 percent from 2000 to 2015.[59] Alcohol use resulted in an additional 3 million deaths in 2016.[58]
Diagnosis
- Further information: Substance use disorder#Diagnosis
The 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) uses the term "substance use disorder" to refer to a spectrum of drug use-related disorders. The DSM-5 eliminates the terms "abuse" and "dependence" from diagnostic categories, instead using the specifiers of mild, moderate and severe to indicate the extent of disordered use. These specifiers are determined by the number of diagnostic criteria present in a given case. In the DSM-5, the term drug addiction is synonymous with severe substance use disorder.[60][3]
The DSM-5 introduced a new diagnostic category for behavioral addictions; however, problem gambling is the only condition included in that category in the 5th edition.[16] Internet gaming disorder is listed as a "condition requiring further study" in the DSM-5.[61]
Past editions have used physical dependence and the associated withdrawal syndrome to identify an addictive state. Physical dependence occurs when the body has adjusted by incorporating the substance into its "normal" functioning – i.e., attains homeostasis – and therefore physical withdrawal symptoms occur upon cessation of use.[62] Tolerance is the process by which the body continually adapts to the substance and requires increasingly larger amounts to achieve the original effects. Withdrawal refers to physical and psychological symptoms experienced when reducing or discontinuing a substance that the body has become dependent on. Symptoms of withdrawal generally include but are not limited to body aches, anxiety, irritability, intense cravings for the substance, nausea, hallucinations, headaches, cold sweats, tremors, and seizures.
Medical researchers who actively study addiction have criticized the DSM classification of addiction for being flawed and involving arbitrary diagnostic criteria.[17] Writing in 2013, the director of the United States National Institute of Mental Health discussed the invalidity of the DSM-5's classification of mental disorders:[63]
While DSM has been described as a "Bible" for the field, it is, at best, a dictionary, creating a set of labels and defining each. The strength of each of the editions of DSM has been "reliability" – each edition has ensured that clinicians use the same terms in the same ways. The weakness is its lack of validity. Unlike our definitions of ischemic heart disease, lymphoma, or AIDS, the DSM diagnoses are based on a consensus about clusters of clinical symptoms, not any objective laboratory measure. In the rest of medicine, this would be equivalent to creating diagnostic systems based on the nature of chest pain or the quality of fever.
Given that addiction manifests in structural changes to the brain, it is possible that non-invasive neuroimaging scans obtained via MRI could be used to help diagnose addiction in the future.[64] As a diagnostic biomarker, ΔFosB expression could be used to diagnose an addiction in humans, but this would require a brain biopsy and therefore is not used in clinical practice.
Individuals whose drug or alcohol use cause significant impairment or distress may have a substance use disorder (SUD).[52] Diagnosis usually involves an in-depth examination, typically by psychiatrist, psychologist, or drug and alcohol counselor.[65] The most commonly used guidelines are published in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).[65] There are 11 diagnostic criteria which can be broadly categorized into issues arising from substance use related to loss of control, strain to one's interpersonal life, hazardous use, and pharmacologic effects.[52]
DSM-5 guidelines for the diagnosis of a substance use disorder requires that the individual have significant impairment or distress from their pattern of drug use, and at least two of the symptoms listed below in a given year.[52]
- Using more of a substance than planned, or using a substance for a longer interval than desired
- Inability to cut down despite desire to do so
- Spending substantial amount of the day obtaining, using, or recovering from substance use
- Cravings or intense urges to use
- Repeated usage causes or contributes to an inability to meet important social, or professional obligations
- Persistent usage despite user's knowledge that it is causing frequent problems at work, school, or home
- Giving up or cutting back on important social, professional, or leisure activities because of use
- Using in physically hazardous situations, or usage causing physical or mental harm
- Persistent use despite the user's awareness that the substance is causing or at least worsening a physical or mental problem
- Tolerance: needing to use increasing amounts of a substance to obtain its desired effects
- Withdrawal: characteristic group of physical effects or symptoms that emerge as amount of substance in the body decreases
There are additional qualifiers and exceptions outlined in the DSM. For instance, if an individual is taking opiates as prescribed, they may experience physiologic effects of tolerance and withdrawal, but this would not cause an individual to meet criteria for a SUD without additional symptoms also being present.[52] A medical professional trained to evaluate and treat substance use disorders will take these nuances into account during a diagnostic evaluation.
Treatment
According to a review, "in order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, twelve-step programs, and residential treatment facilities."[6]
Detoxification
Depending on the severity of use, and the given substance, early treatment of acute withdrawal may include medical detoxification. Of note, acute withdrawal from heavy alcohol use should be done under medical supervision to prevent a potentially deadly withdrawal syndrome known as delirium tremens. See also Alcohol detoxification.
Therapy
Therapists often classify people with chemical dependencies as either interested or not interested in changing. About 11% of Americans with substance use disorder seek treatment, and 40–60% of those people relapse within a year.[66] Treatments usually involve planning for specific ways to avoid the addictive stimulus, and therapeutic interventions intended to help a client learn healthier ways to find satisfaction. Clinical leaders in recent years have attempted to tailor intervention approaches to specific influences that affect addictive behavior, using therapeutic interviews in an effort to discover factors that led a person to embrace unhealthy, addictive sources of pleasure or relief from pain.
| Treatments | ||
|---|---|---|
| Behavioral pattern | Intervention | Goals |
| Low self-esteem, anxiety, verbal hostility | Relationship therapy, client centered approach | Increase self-esteem, reduce hostility and anxiety |
| Defective personal constructs, ignorance of interpersonal means | Cognitive restructuring including directive and group therapies | Insight |
| Focal anxiety such as fear of crowds | Desensitization | Change response to same cue |
| Undesirable behaviors, lacking appropriate behaviors | Aversive conditioning, operant conditioning, counter conditioning | Eliminate or replace behavior |
| Lack of information | Provide information | Have client act on information |
| Difficult social circumstances | Organizational intervention, environmental manipulation, family counseling | Remove cause of social difficulty |
| Poor social performance, rigid interpersonal behavior | Sensitivity training, communication training, group therapy | Increase interpersonal repertoire, desensitization to group functioning |
| Grossly bizarre behavior | Medical referral | Protect from society, prepare for further treatment |
| Adapted from: Essentials of Clinical Dependency Counseling, Aspen Publishers | ||
From the applied behavior analysis literature and the behavioral psychology literature, several evidence-based intervention programs have emerged, such as behavioral marital therapy, community reinforcement approach, cue exposure therapy, and contingency management strategies.[67][68] In addition, the same author suggests that social skills training adjunctive to inpatient treatment of alcohol dependence is probably efficacious.
Medication
Medication-assisted treatment (MAT) refers to the combination of behavioral interventions and medications to treat substance use disorders.[69] Certain medications can be useful in treating severe substance use disorders. In the United States five medications are approved to treat alcohol and opioid use disorders.[70] There are no approved medications for cocaine, methamphetamine, or other substance use disorders as of 2002.[70]
Medications, such as methadone and disulfiram, can be used as part of broader treatment plans to help a patient function comfortably without illicit opioids or alcohol.[71] Medications can be used in treatment to lessen withdrawal symptoms. Evidence has demonstrated the efficacy of MAT at reducing illicit drug use and overdose deaths, improving retention in treatment, and reducing HIV transmission.[72][73][74]
Behavioral therapy
A meta-analytic review on the efficacy of various behavioral therapies for treating drug and behavioral addictions found that cognitive behavioral therapy (e.g., relapse prevention and contingency management), motivational interviewing, and a community reinforcement approach were effective interventions with moderate effect sizes.[75]
Clinical and preclinical evidence indicate that consistent aerobic exercise, especially endurance exercise (e.g., marathon running), actually prevents the development of certain drug addictions and is an effective adjunct treatment for drug addiction, and for psychostimulant addiction in particular.[13][76][77][78][79] Consistent aerobic exercise magnitude-dependently (i.e., by duration and intensity) reduces drug addiction risk, which appears to occur through the reversal of drug induced addiction-related neuroplasticity.[13][77] One review noted that exercise may prevent the development of drug addiction by altering ΔFosB or c-Fos immunoreactivity in the striatum or other parts of the reward system.[79] Aerobic exercise decreases drug self-administration, reduces the likelihood of relapse, and induces opposite effects on striatal dopamine receptor D2 (DRD2) signaling (increased DRD2 density) to those induced by addictions to several drug classes (decreased DRD2 density).[13][77] Consequently, consistent aerobic exercise may lead to better treatment outcomes when used as an adjunct treatment for drug addiction.[13][77][78]
Medication
Alcohol addiction
- Further information: Alcoholism
Alcohol, like opioids, can induce a severe state of physical dependence and produce withdrawal symptoms such as delirium tremens. Because of this, treatment for alcohol addiction usually involves a combined approach dealing with dependence and addiction simultaneously. Benzodiazepines have the largest and the best evidence base in the treatment of alcohol withdrawal and are considered the gold standard of alcohol detoxification.[80]
Pharmacological treatments for alcohol addiction include drugs like naltrexone (opioid antagonist), disulfiram, acamprosate, and topiramate.[81][82] Rather than substituting for alcohol, these drugs are intended to affect the desire to drink, either by directly reducing cravings as with acamprosate and topiramate, or by producing unpleasant effects when alcohol is consumed, as with disulfiram. These drugs can be effective if treatment is maintained, but compliance can be an issue as alcoholic patients often forget to take their medication, or discontinue use because of excessive side effects.[83][84] According to a Cochrane Collaboration review, the opioid antagonist naltrexone has been shown to be an effective treatment for alcoholism, with the effects lasting three to twelve months after the end of treatment.[85]
Cannabinoid addiction
As of 2010, there are no effective pharmacological interventions for cannabinoid addiction.[86] A 2013 review on cannabinoid addiction noted that the development of CB1 receptor agonists that have reduced interaction with β-arrestin 2 signaling might be therapeutically useful.[87]
Nicotine addiction
- Further information: Smoking cessation
Another area in which drug treatment has been widely used is in the treatment of nicotine addiction, which usually involves the use of nicotine replacement therapy, nicotinic receptor antagonists, or nicotinic receptor partial agonists.[88][89] Examples of drugs that act on nicotinic receptors and have been used for treating nicotine addiction include antagonists like bupropion and the partial agonist varenicline.[88][89]
Opioid addiction
- Further information: Opioid use disorder
Opioids cause physical dependence, and treatment typically addresses both dependence and addiction.
Physical dependence is treated using replacement drugs such as suboxone or subutex (both containing the active ingredients buprenorphine) and methadone.[90][91] Although these drugs perpetuate physical dependence, the goal of opiate maintenance is to provide a measure of control over both pain and cravings. Use of replacement drugs increases the addicted individual's ability to function normally and eliminates the negative consequences of obtaining controlled substances illicitly. Once a prescribed dosage is stabilized, treatment enters maintenance or tapering phases. In the United States, opiate replacement therapy is tightly regulated in methadone clinics and under the DATA 2000 legislation. In some countries, other opioid derivatives such as levomethadyl acetate,[92] dihydrocodeine,[93] dihydroetorphine[94] and even heroin[95][96] are used as substitute drugs for illegal street opiates, with different prescriptions being given depending on the needs of the individual patient. Baclofen has led to successful reductions of cravings for stimulants, alcohol, and opioids, and also alleviates alcohol withdrawal syndrome. Many patients have stated they "became indifferent to alcohol" or "indifferent to cocaine" overnight after starting baclofen therapy.[97] Some studies show the interconnection between opioid drug detoxification and overdose mortality.[98]
Psychostimulant addiction
As of May 2014, there is no effective pharmacotherapy for any form of psychostimulant addiction.[6][99][100][101] Reviews from 2015, 2016, and 2018 indicated that TAAR1-selective agonists have significant therapeutic potential as a treatment for psychostimulant addictions;[102][103][104] however, as of 2018, the only compounds which are known to function as TAAR1-selective agonists are experimental drugs.[102][103][104]
Research
Template:Expand section Research indicates that vaccines which utilize anti-drug monoclonal antibodies can mitigate drug-induced positive reinforcement by preventing the drug from moving across the blood–brain barrier;[105] however, current vaccine-based therapies are only effective in a relatively small subset of individuals.[105][106] As of November 2015, vaccine-based therapies are being tested in human clinical trials as a treatment for addiction and preventive measure against drug overdoses involving nicotine, cocaine, and methamphetamine.[105]
The new study shows, that the vaccine may also save lives during a drug overdose. In this instance, the idea is that the body will respond to the vaccine by quickly producing antibodies to prevent the opioids from accessing the brain.[107]
Since addiction involves abnormalities in glutamate and GABAergic neurotransmission,[108][109] receptors associated with these neurotransmitters (e.g., AMPA receptors, NMDA receptors, and GABAB receptors) are potential therapeutic targets for addictions.[108][109][110][111] N-acetylcysteine, which affects metabotropic glutamate receptors and NMDA receptors, has shown some benefit in preclinical and clinical studies involving addictions to cocaine, heroin, and cannabinoids.[108] It may also be useful as an adjunct therapy for addictions to amphetamine-type stimulants, but more clinical research is required.[108]
Current medical reviews of research involving lab animals have identified a drug class – class I histone deacetylase inhibitors[note 2] – that indirectly inhibits the function and further increases in the expression of accumbal ΔFosB by inducing G9a expression in the nucleus accumbens after prolonged use.[114][115][112][113] These reviews and subsequent preliminary evidence which used oral administration or intraperitoneal administration of the sodium salt of butyric acid or other class I HDAC inhibitors for an extended period indicate that these drugs have efficacy in reducing addictive behavior in lab animals[note 3] that have developed addictions to ethanol, psychostimulants (i.e., amphetamine and cocaine), nicotine, and opiates;[115][113][116][117] however, few clinical trials involving human addicts and any HDAC class I inhibitors have been conducted to test for treatment efficacy in humans or identify an optimal dosing regimen.[note 4]
Gene therapy for addiction is an active area of research. One line of gene therapy research involves the use of viral vectors to increase the expression of dopamine D2 receptor proteins in the brain.[119][120][121][122][123]
Risk factors
There are many known risk factors associated with an increased chance of developing a substance use disorder. Children born to parents with SUDs have roughly a two-fold increased risk in developing a SUD compared to children born to parents without any SUDs.[124] Taking highly addictive drugs, and those who develop SUDs in their teens are more likely to have continued symptoms into adulthood.[124] Other common risk factors are being male, being under 25, having other mental health problems, and lack of familial support and supervision.[124] Psychological risk factors include high impulsivity, sensation seeking, neuroticism and openness to experience in combination with low conscientiousness.[125][126]
There are a number of genetic and environmental risk factors for developing an addiction, that vary across the population.[1][127] Genetic and environmental risk factors each account for roughly half of an individual's risk for developing an addiction;[1] the contribution from epigenetic risk factors to the total risk is unknown.[127] Even in individuals with a relatively low genetic risk, exposure to sufficiently high doses of an addictive drug for a long period of time (e.g., weeks–months) can result in an addiction.[1]
Genetic factors
- See also: Alcoholism#Genetic variation
It has long been established that genetic factors along with environmental (e.g., psychosocial) factors are significant contributors to addiction vulnerability.[1][127] Epidemiological studies estimate that genetic factors account for 40–60% of the risk factors for alcoholism.[128] Similar rates of heritability for other types of drug addiction have been indicated by other studies.[129] Knestler hypothesized in 1964 that a gene or group of genes might contribute to predisposition to addiction in several ways. For example, altered levels of a normal protein due to environmental factors could then change the structure or functioning of specific brain neurons during development. These altered brain neurons could change the susceptibility of an individual to an initial drug use experience. In support of this hypothesis, animal studies have shown that environmental factors such as stress can affect an animal's genotype.[129]
Overall, the data implicating specific genes in the development of drug addiction is mixed for most genes. One reason for this may be that the case is due to a focus of current research on common variants. Many addiction studies focus on common variants with an allele frequency of greater than 5% in the general population; however, when associated with disease, these only confer a small amount of additional risk with an odds ratio of 1.1–1.3 percent. On the other hand, the rare variant hypothesis states that genes with low frequencies in the population (<1%) confer much greater additional risk in the development of the disease.[130]
Genome-wide association studies (GWAS) are used to examine genetic associations with dependence, addiction, and drug use. These studies employ an unbiased approach to finding genetic associations with specific phenotypes and give equal weight to all regions of DNA, including those with no ostensible relationship to drug metabolism or response. These studies rarely identify genes from proteins previously described via animal knockout models and candidate gene analysis. Instead, large percentages of genes involved in processes such as cell adhesion are commonly identified. This is not to say that previous findings, or the GWAS findings, are erroneous. The important effects of endophenotypes are typically not capable of being captured by these methods. Furthermore, genes identified in GWAS for drug addiction may be involved either in adjusting brain behavior prior to drug experiences, subsequent to them, or both.[131]
A study that highlights the significant role genetics play in addiction is the twin studies. Twins have similar and sometimes identical genetics. Analyzing these genes in relation to genetics has helped geneticists understand how much of a role genes play in addiction. Studies performed on twins found that rarely did only one twin have an addiction. In most cases where at least one twin suffered from an addiction, both did, and often to the same substance.[132] Cross addiction is when already has a predisposed addiction and then starts to become addicted to something different. If one family member has a history of addiction, the chances of a relative or close family developing those same habits are much higher than one who has not been introduced to addiction at a young age.[133] In a recent study done by the National Institute on Drug Abuse, from 2002 to 2017, overdose deaths have almost tripled amongst male and females. In 2017, 72,306 overdose deaths happened in the U.S. that were reported.[134]
Environmental factors
Environmental risk factors for addiction are the experiences of an individual during their lifetime that interact with the individual's genetic composition to increase or decrease his or her vulnerability to addiction.[1] A number of different environmental factors have been implicated as risk factors for addiction, including various psychosocial stressors;[1] however, an individual's exposure to an addictive drug is by far the most significant environmental risk factor for addiction.[1] The National Institute on Drug Abuse (NIDA) cites lack of parental supervision, the prevalence of peer substance use, drug availability, and poverty as risk factors for substance use among children and adolescents.[135]
Adverse childhood experiences (ACEs) are various forms of maltreatment and household dysfunction experienced in childhood. The Adverse Childhood Experiences Study by the Centers for Disease Control and Prevention has shown a strong dose–response relationship between ACEs and numerous health, social, and behavioral problems throughout a person's lifespan, including those associated with substance abuse.[136] Children's neurological development can be permanently disrupted when they are chronically exposed to stressful events such as physical, emotional, or sexual abuse, physical or emotional neglect, witnessing violence in the household, or a parent being incarcerated or suffering from a mental illness. As a result, the child's cognitive functioning or ability to cope with negative or disruptive emotions may be impaired. Over time, the child may adopt substance use as a coping mechanism, particularly during adolescence.[136] A study of 900 court cases involving children who experienced abuse found that a vast amount of them went on to suffer from some form of addiction in their adolescence or adult life.[137] This pathway towards addiction that is opened through stressful experiences during childhood can be avoided by a change in environmental factors throughout an individual's life and opportunities of professional help.[137] If one has friends or peers who engage in drug use favorably, the chances of them developing an addiction increases. Family conflict and home management is also a cause for one to become engaged in alcohol or other drug use.[138]
Age
Adolescence represents a period of unique vulnerability for developing an addiction.[139] In adolescence, the incentive-rewards systems in the brain mature well before the cognitive control center. This consequentially grants the incentive-rewards systems a disproportionate amount of power in the behavioral decision-making process. Therefore, adolescents are increasingly likely to act on their impulses and engage in risky, potentially addicting behavior before considering the consequences.[140] Not only are adolescents more likely to initiate and maintain drug use, but once addicted they are more resistant to treatment and more liable to relapse.[141][142]
Statistics have shown that those who start to drink alcohol at a younger age are more likely to become dependent later on. About 33% of the population tasted their first alcohol between the ages of 15 and 17, while 18% experienced it prior to this. As for alcohol abuse or dependence, the numbers start off high with those who first drank before they were 12 and then drop off after that. For example, 16% of alcoholics began drinking prior to turning 12 years old, while only 9% first touched alcohol between 15 and 17. This percentage is even lower, at 2.6%, for those who first started the habit after they were 21.[143]
Most individuals are exposed to and use addictive drugs for the first time during their teenage years.[144] In the United States, there were just over 2.8 million new users of illicit drugs in 2013 (~7,800 new users per day);[144] among them, 54.1% were under 18 years of age.[144] In 2011, there were approximately 20.6 million people in the United States over the age of 12 with an addiction.[145] Over 90% of those with an addiction began drinking, smoking or using illicit drugs before the age of 18.[145]
Comorbid disorders
Individuals with comorbid (i.e., co-occurring) mental health disorders such as depression, anxiety, attention-deficit/hyperactivity disorder (ADHD) or post-traumatic stress disorder are more likely to develop substance use disorders.[146][147][148] The NIDA cites early aggressive behavior as a risk factor for substance use.[135] A study by the National Bureau of Economic Research found that there is a "definite connection between mental illness and the use of addictive substances" and a majority of mental health patients participate in the use of these substances: 38% alcohol, 44% cocaine, and 40% cigarettes.[149]
Epigenetic factors
Transgenerational epigenetic inheritance
Epigenetic genes and their products (e.g., proteins) are the key components through which environmental influences can affect the genes of an individual;[127] they also serve as the mechanism responsible for transgenerational epigenetic inheritance, a phenomenon in which environmental influences on the genes of a parent can affect the associated traits and behavioral phenotypes of their offspring (e.g., behavioral responses to environmental stimuli).[127] In addiction, epigenetic mechanisms play a central role in the pathophysiology of the disease;[1] it has been noted that some of the alterations to the epigenome which arise through chronic exposure to addictive stimuli during an addiction can be transmitted across generations, in turn affecting the behavior of one's children (e.g., the child's behavioral responses to addictive drugs and natural rewards).[127][150]
The general classes of epigenetic alterations that have been implicated in transgenerational epigenetic inheritance include DNA methylation, histone modifications, and downregulation or upregulation of microRNAs.[127] With respect to addiction, more research is needed to determine the specific heritable epigenetic alterations that arise from various forms of addiction in humans and the corresponding behavioral phenotypes from these epigenetic alterations that occur in human offspring.[127][150] Based upon preclinical evidence from animal research, certain addiction-induced epigenetic alterations in rats can be transmitted from parent to offspring and produce behavioral phenotypes that decrease the offspring's risk of developing an addiction.[note 5][127] More generally, the heritable behavioral phenotypes that are derived from addiction-induced epigenetic alterations and transmitted from parent to offspring may serve to either increase or decrease the offspring's risk of developing an addiction.[127][150]
Epidemiology
Due to cultural variations, the proportion of individuals who develop a drug or behavioral addiction within a specified time period (i.e., the prevalence) varies over time, by country, and across national population demographics (e.g., by age group, socioeconomic status, etc.).[127]
Asia
The prevalence of alcohol dependence is not as high as is seen in other regions. In Asia, not only socioeconomic factors but also biological factors influence drinking behavior.[151]
The overall prevalence of smartphone ownership is 62%, ranging from 41% in China to 84% in South Korea. Moreover, participation in online gaming ranges from 11% in China to 39% in Japan. Hong Kong has the highest number of adolescents reporting daily or above Internet use (68%). Internet addiction disorder is highest in the Philippines, according to both the IAT (Internet Addiction Test) – 5% and the CIAS-R (Revised Chen Internet Addiction Scale) – 21%.[152]
Australia
The prevalence of substance abuse disorder among Australians was reported at 5.1% in 2009.[153]
Europe
In 2015, the estimated prevalence among the adult population was 18.4% for heavy episodic alcohol use (in the past 30 days); 15.2% for daily tobacco smoking; and 3.8, 0.77, 0.37 and 0.35% in 2017 cannabis, amphetamine, opioid and cocaine use. The mortality rates for alcohol and illicit drugs were highest in Eastern Europe.[154]
United States
Based upon representative samples of the US youth population in 2011, the lifetime prevalence[note 6] of addictions to alcohol and illicit drugs has been estimated to be approximately 8% and 2–3% respectively.[19] Based upon representative samples of the US adult population in 2011, the 12 month prevalence of alcohol and illicit drug addictions were estimated at roughly 12% and 2–3% respectively.[19] The lifetime prevalence of prescription drug addictions is currently around 4.7%.[155]
As of 2016 about 22 million people in the United States need treatment for an addiction to alcohol, nicotine, or other drugs.[20][156] Only about 10%, or a little over 2 million, receive any form of treatments, and those that do generally do not receive evidence-based care.[20][156] One-third of inpatient hospital costs and 20% of all deaths in the US every year are the result of untreated addictions and risky substance use.[20][156] In spite of the massive overall economic cost to society, which is greater than the cost of diabetes and all forms of cancer combined, most doctors in the US lack the training to effectively address a drug addiction.[20][156]
Another review listed estimates of lifetime prevalence rates for several behavioral addictions in the United States, including 1–2% for compulsive gambling, 5% for sexual addiction, 2.8% for food addiction, and 5–6% for compulsive shopping.[13] A systematic review indicated that the time-invariant prevalence rate for sexual addiction and related compulsive sexual behavior (e.g., compulsive masturbation with or without pornography, compulsive cybersex, etc.) within the United States ranges from 3–6% of the population.[33]
According to a 2017 poll conducted by the Pew Research Center, almost half of US adults know a family member or close friend who has struggled with a drug addiction at some point in their life.[157]
In 2019, opioid addiction was acknowledged as a national crisis in the United States.[158] A Washington Post article stated that "America’s largest drug companies flooded the country with pain pills from 2006 through 2012, even when it became apparent that they were fueling addiction and overdoses."
South America
The realities of opioid use and abuse in Latin America may be deceptive if observations are limited to epidemiological findings. In the United Nations Office on Drugs and Crime report,[159] although South America produced 3% of the world's morphine and heroin and 0.01% of its opium, prevalence of use is uneven. According to the Inter-American Commission on Drug Abuse Control, consumption of heroin is low in most Latin American countries, although Colombia is the area's largest opium producer. Mexico, because of its border with the United States, has the highest incidence of use.[160]
Notes
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Cite error: Invalid
<ref>tag; no text was provided for refs namedCellular basis - ↑ 2.0 2.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedNestler Labs Glossary - ↑ 3.0 3.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedBrain disease - ↑ (October 2008) The disease of addiction: origins, treatment, and recovery. Disease-a-Month 54 (10): 696–721.
- ↑ 5.0 5.1 (2009) "Chapter 15: Reinforcement and Addictive Disorders", Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 2nd, New York: McGraw-Hill Medical, 364–65, 375. ISBN 978-0-07-148127-4. “The defining feature of addiction is compulsive, out-of-control drug use, despite negative consequences. ...
compulsive eating, shopping, gambling, and sex – so-called "natural addictions" – Indeed, addiction to both drugs and behavioral rewards may arise from similar dysregulation of the mesolimbic dopamine system.” - ↑ 6.0 6.1 6.2 6.3 (February 2013) The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans. Subst. Abuse Rehabil. 4: 29–43.
- ↑ [1][2][3][4][5][6]
- ↑ American Society for Addiction Medicine (2012). Definition of Addiction.
- ↑ (August 1992) The definition of alcoholism. The Joint Committee of the National Council on Alcoholism and Drug Dependence and the American Society of Addiction Medicine to Study the Definition and Criteria for the Diagnosis of Alcoholism. JAMA 268 (8): 1012–14.
- ↑ (1988). Addictive behaviors: etiology and treatment. Annu Rev Psychol 39: 223–52.
- ↑ Holden, Constance (2001-11-02). 'Behavioral' Addictions: Do They Exist?. Science 294 (5544): 980–982.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 (November 2011) Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. 12 (11): 623–637.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 13.12 13.13 13.14 13.15 13.16 13.17 13.18 Olsen CM (December 2011). Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 61 (7): 1109–22.
- ↑ 14.0 14.1 14.2 (2012). Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms. Journal of Psychoactive Drugs 44 (1): 38–55.
- ↑ Meredith E. Gansner, M. D. (2019-09-12). Gaming Addiction in ICD-11: Issues and Implications (in en).
- ↑ 16.0 16.1 American Psychiatric Association (2013). Substance-Related and Addictive Disorders pp. 1–2. American Psychiatric Publishing. Archived from the original on 15 August 2015. Retrieved 10 July 2015.
- ↑ 17.0 17.1 (2015) "Chapter 16: Reinforcement and Addictive Disorders", Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 3rd, New York: McGraw-Hill Medical. ISBN 978-0-07-182770-6. “The official diagnosis of drug addiction by the Diagnostic and Statistic Manual of Mental Disorders (2013), which uses the term substance use disorder, is flawed. Criteria used to make the diagnosis of substance use disorders include tolerance and somatic dependence/withdrawal, even though these processes are not integral to addiction as noted. It is ironic and unfortunate that the manual still avoids use of the term addiction as an official diagnosis, even though addiction provides the best description of the clinical syndrome.”
- ↑ 18.0 18.1 (2009) "Chapter 1: Basic Principles of Neuropharmacology", Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 2nd, New York: McGraw-Hill Medical. ISBN 978-0-07-148127-4. “Drug abuse and addiction exact an astoundingly high financial and human toll on society through direct adverse effects, such as lung cancer and hepatic cirrhosis, and indirect adverse effects –for example, accidents and AIDS – on health and productivity.”
- ↑ 19.0 19.1 19.2 19.3 (June 2012) Epidemiology of Substance Use Disorders. Hum. Genet. 131 (6): 779–89.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 American Board of Medical Specialties recognizes the new subspecialty of addiction medicine (14 March 2016). Retrieved 3 April 2016.
- ↑ (2013) "Economic consequences of drug abuse", International Narcotics Control Board Report: 2013. United Nations – International Narcotics Control Board. ISBN 978-92-1-148274-4. Retrieved 28 September 2018.
- ↑ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 29: 565–598.
- ↑ (January 2013) Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants. Prog. Neurobiol. 100: 60–80.
- ↑ Kanehisa Laboratories (2 August 2013). Alcoholism – Homo sapiens (human). Retrieved 10 April 2014.
- ↑ (February 2013) Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J. Neurosci. 33 (8): 3434–42.
- ↑ 26.0 26.1 (2007). Reward system and addiction: What dopamine does and doesn't do. Current Opinion in Pharmacology 7 (1): 69–76.
- ↑ (2008). The neurobiology and genetics of impulse control disorders: Relationships to drug addictions. Biochemical Pharmacology 75 (1): 63–75.
- ↑ (2004). The Neurobiology of Dopamine Signaling. Archives of Neurology 61 (5): 641–4.
- ↑ (2007). Diagnostic instruments for behavioural addiction: an overview. Psychosom Med 4: Doc11.
- ↑ Potenza MN (September 2006). Should addictive disorders include non-substance-related conditions?. Addiction 101 Suppl 1: 142–51.
- ↑ (1996). Understanding the means and objects of addiction: Technology, the internet, and gambling. Journal of Gambling Studies 12 (4): 461–9.
- ↑ (31 August 2009) Textbook of Anxiety Disorders. American Psychiatric Pub, 359–. ISBN 978-1-58562-254-2. Retrieved 24 April 2010.
- ↑ 33.0 33.1 33.2 33.3 (2014). Sexual addiction or hypersexual disorder: different terms for the same problem? A review of the literature. Curr. Pharm. Des. 20 (25): 4012–20.
- ↑ 34.0 34.1 34.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedAmph-Sex X-sensitization through D1 signaling - ↑ 35.0 35.1 35.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedAmph-Sex X-sensitization through NMDA signaling - ↑ 36.0 36.1 36.2 (September 2010) Introduction to behavioral addictions. Am. J. Drug Alcohol Abuse 36 (5): 233–241.
- ↑ (2013). Internet gaming addiction: current perspectives. Psychology Research and Behavior Management 6 (6): 125–137.
- ↑ Is your child a gaming addict?.
- ↑ Grant, Jon: Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions
- ↑ (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience 13 (5): 635–41.
- ↑ American Society of Addiction Medicine. Public Policy Statement: Definition of Addiction. https://www.asam.org/resources/definition-of-addiction
- ↑ (2012). Abnormal White Matter Integrity in Adolescents with Internet Addiction Disorder: A Tract-Based Spatial Statistics Study. PLoS ONE 7 (1): e30253.
- ↑ (2011). Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: An fMRI study during a guessing task. Journal of Psychiatric Research 45 (11): 1525–9.
- ↑ (2011). Male Internet addicts show impaired executive control ability: Evidence from a color-word Stroop task. Neuroscience Letters 499 (2): 114–8.
- ↑ (2011). Microstructure Abnormalities in Adolescents with Internet Addiction Disorder. PLoS ONE 6 (6): e20708.
- ↑ (2011). Reduced striatal dopamine D2 receptors in people with Internet addiction. NeuroReport 22 (8): 407–11.
- ↑ (2011). Functional magnetic resonance imaging of brain of college students with internet addiction. 中南大学学报 (医学版) [Journal of Central South University (Medical sciences)] 36 (8): 744–9.
- ↑ (2011). The effect of psychiatric symptoms on the internet addiction disorder in Isfahan's University students. Journal of Research in Medical Sciences 16 (6): 793–800.
- ↑ Craving Facebook? UAlbany Study Finds Social Media to be Potentially Addictive, Associated with Substance Abuse.
- ↑ (2013) Diagnostic and statistical manual of mental disorders., 5, Arlington, VA: American Psychiatric Association. ISBN 978-0-89042-554-1. OCLC 830807378.
- ↑ NAMI Comments on the APA's Dr aft Revision of the DSM-V Substance Use Disorders. National Alliance on Mental Illness.
- ↑ 52.0 52.1 52.2 52.3 52.4 (2013) Diagnostic and statistical manual of mental disorders., 5, American Psychiatric Association. ISBN 978-0-89042-554-1.
- ↑ Substance Abuse and Mental Health Services Administration (June 2016). Substance Use Disorders. Substance Abuse and Mental Health Services Administration (US).
- ↑ Guha, Martin (2014-03-11). Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th edition). Reference Reviews 28 (3).
- ↑ (August 2013) DSM-5 criteria for substance use disorders: recommendations and rationale. The American Journal of Psychiatry 170 (8): 834–51.
- ↑ World Health Organization, ICD-11 for Mortality and Morbidity Statistics (ICD-11 MMS), 2018 version for preparing implementation, rev. April 2019
- ↑ 57.0 57.1 57.2 World Drug Report 2019: 35 million people worldwide suffer from drug use disorders while only 1 in 7 people receive treatment.
- ↑ 58.0 58.1 (2018) Global status report on alcohol and health 2018. WHO. Retrieved 3 May 2020.
- ↑ Prelaunch.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedSurgeon General severe SUD - ↑ (September 2014) An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction 109 (9): 1399–406.
- ↑ (1999). Drugs of abuse and brain gene expression. Psychosom Med 61 (5): 630–50.
- ↑ Transforming Diagnosis. National Institute of Mental Health. Retrieved 17 June 2015.
- ↑ (2019). Substance Abuse and White Matter: Findings, Limitations, and Future of Diffusion Tensor Imaging Research. Drug and Alcohol Dependence 197 (4): 288–298.
- ↑ 65.0 65.1 Drug addiction (substance use disorder) – Symptoms and causes.
- ↑ (October 2000)Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284 (13): 1689–95.
- ↑ (2006). Evidence-Based Practice in Psychology and Behavior Analysis. The Behavior Analyst Today 7 (3): 335–350.
- ↑ (1998). An update on empirically validated therapies. Clinical Psychology 49: 5–14.
- ↑ (July 2012) Opioid addiction and abuse in primary care practice: a comparison of methadone and buprenorphine as treatment options. Journal of the National Medical Association 104 (7–8): 342–50.
- ↑ 70.0 70.1 American Psychiatric Association. (2002). American Psychiatric Association practice guidelines for the treatment of psychiatric disorders.. The Association. ISBN 0-89042-320-2. OCLC 48656105.
- ↑ Massachusetts. Center for Health Information and Analysis, issuing body.. Access to substance use disorder treatment in Massachusetts. OCLC 911187572.
- ↑ Holt, Dr. Harry (2019-07-15). Stigma Associated with Opioid Use Disorder and Medication Assisted Treatment.
- ↑ (May 2013) Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995-2009. American Journal of Public Health 103 (5): 917–22.
- ↑ Administration (US), Substance Abuse and Mental Health Services (November 2016). EARLY INTERVENTION, TREATMENT, AND MANAGEMENT OF SUBSTANCE USE DISORDERS. US Department of Health and Human Services.
- ↑ (2015). [Psychosocial Treatment of Addictive Disorders – An Overview of Psychotherapeutic Options and their Efficacy]. Fortschr Neurol Psychiatr 83 (4): 201–10.
- ↑ (February 2016) Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments. Front. Psychiatry 6: 175.
- ↑ 77.0 77.1 77.2 77.3 (September 2013) Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev 37 (8): 1622–44.
- ↑ 78.0 78.1 (2015). Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am J Drug Alcohol Abuse 41 (1): 7–15.
- ↑ 79.0 79.1 (July 2015) Sex differences in drug addiction and response to exercise intervention: From human to animal studies. Front. Neuroendocrinol. 40: 24–41.
- ↑ Sachdeva A, Choudhary M, Chandra M. (Sep 2015) "Alcohol Withdrawal Syndrome: Benzodiazepines and Beyond." PMID: 26500991
- ↑ (December 2006) New pharmacological approaches for the treatment of alcoholism. Expert Opinion on Pharmacotherapy 7 (17): 2341–53.
- ↑ (October 2006) Choosing the right medication for the treatment of alcoholism. Current Psychiatry Reports 8 (5): 383–88.
- ↑ (July 2004) Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 99 (7): 811–28.
- ↑ (November 2005) Medications for treating alcohol dependence. American Family Physician 72 (9): 1775–80.
- ↑ (December 2010) Opioid antagonists for alcohol dependence. The Cochrane Database of Systematic Reviews (12): CD001867.
- ↑ (April 2010) Cognitive function as an emerging treatment target for marijuana addiction. Exp Clin Psychopharmacol 18 (2): 109–19.
- ↑ (August 2013) Molecular mechanisms of cannabinoid addiction. Curr. Opin. Neurobiol. 23 (4): 487–92.
- ↑ 88.0 88.1 (2007). Emerging pharmacotherapies for smoking cessation. Am J Health Syst Pharm 64 (16): 1693–98.
- ↑ 89.0 89.1 (2014) Nicotinic receptor antagonists as treatments for nicotine abuse, Advances in Pharmacology, 513–51. DOI:10.1016/B978-0-12-420118-7.00013-5. ISBN 978-0-12-420118-7.
- ↑ (2000). A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N. Engl. J. Med. 343 (18): 1290–97.
- ↑ (2007). Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess 11 (9): 1–171, iii–iv.
- ↑ (2005). Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence. Exp Clin Psychopharmacol 13 (4): 293–302.
- ↑ (2006). Addressing the efficacy of dihydrocodeine versus methadone as an alternative maintenance treatment for opiate dependence: A randomized controlled trial. Addiction 101 (12): 1752–59.
- ↑ Qin Bo-Yi (1998). Advances in dihydroetorphine: From analgesia to detoxification. Drug Development Research 39 (2): 131–34. Link
- ↑ (1998). Feasibility of prescribing injectable heroin and methadone to opiate-dependent drug users: associated health gains and harm reductions. Med. J. Aust. 168 (12): 596–600.
- ↑ (2007). Pathways into receiving a prescription for diamorphine (heroin) for the treatment of opiate dependence in the United kingdom. Eur Addict Res 13 (3): 144–47.
- ↑ (2007). Pharmacotherapy of dual substance abuse and dependence. CNS Drugs 21 (3): 213–37.
- ↑ Strang J, McCambridge J. (May 2003) "Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study" PMID: 12727768
- ↑ (May 2014) Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol 7 (3): 363–74.
- ↑ (September 2013) Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst. Rev. 9 (9): CD009695.
- ↑ (February 2014) Future pharmacological treatments for substance use disorders. Br. J. Clin. Pharmacol. 77 (2): 382–400.
- ↑ 102.0 102.1 (February 2016) "TAARgeting Addiction" – The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference. Drug Alcohol Depend. 159: 9–16.
- ↑ 103.0 103.1 (August 2015) Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction. Eur. J. Pharmacol. 761: 345–52.
- ↑ 104.0 104.1 (December 2018) Drug addiction: a curable mental disorder?. Acta Pharmacologica Sinica 39 (12): 1823–1829.
- ↑ 105.0 105.1 105.2 (November 2015) Is immunotherapy an opportunity for effective treatment of drug addiction?. Vaccine 33 (48): 6545–51.
- ↑ (June 2015) The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. J. Immunol. 194 (12): 5926–36.
- ↑ Painter A. (2019) "Researchers working to develop vaccines to fight opioid addiction" Vtnews.vt.edu
- ↑ 108.0 108.1 108.2 108.3 (March 2016) Advances and challenges in pharmacotherapeutics for amphetamine-type stimulants addiction. Eur. J. Pharmacol. 780: 129–35.
- ↑ 109.0 109.1 (February 2016) Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: Relationships to resting-state functional connectivity. Neurosci Biobehav Rev 61: 35–52.
- ↑ (April 2015) [GABAB receptor as therapeutic target for drug addiction: from baclofen to positive allosteric modulators]. Psychiatr. Pol. 49 (2): 215–23.
- ↑ (January 2015) GABAB receptors as a therapeutic strategy in substance use disorders: focus on positive allosteric modulators. Neuropharmacology 88: 36–47.
- ↑ 112.0 112.1 (February 2015) The Epigenetic Mechanisms of Amphetamine. J. Addict. Prev. 2015 (Suppl 1).
- ↑ 113.0 113.1 113.2 113.3 (February 2015) Regulation of chromatin states by drugs of abuse. Curr. Opin. Neurobiol. 30: 112–21.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedG9a reverses ΔFosB plasticity - ↑ 115.0 115.1 115.2 (January 2014) Epigenetic mechanisms of drug addiction. Neuropharmacology 76 Pt B: 259–68.
- ↑ 116.0 116.1 Primary references involving sodium butyrate:
Template:Bull (April 2013) Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat. Neurosci. 16 (4): 434–40.
Template:Bull (July 2015) The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals. Addict Biol 20 (4): 676–89.
Template:Bull (April 2015) Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats. PLOS One 10 (4): e0124796. - ↑ (August 2015) Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci. Lett. 601: 11–19.
- ↑ (2016) Effect of add-on valproate on craving in methamphetamine depended patients: A randomized trial. Advanced Biomedical Research 5: 149.
- ↑ Gene Therapy For Addiction: Flooding Brain With 'Pleasure Chemical' Receptors Works On Cocaine, As On Alcohol.
- ↑ Abuse, National Institute on Drug (14 January 2016). Gene Transfer Therapy for Cocaine Addiction Passes Tests in Animals.
- ↑ (2014). Physiologic and metabolic safety of butyrylcholinesterase gene therapy in mice. Vaccine 32 (33): 4155–62.
- ↑ Using Adeno-Associated Virus (AAV) Mediated Sustained Expression of an Anti-methamphetamine Antibody Fragment to Alter Methamphetamine Disposition in Mice.
- ↑ ATTC – Addiction Science Made Easy.
- ↑ 124.0 124.1 124.2 Ferri, Fred (2019). Ferri's Clinical Advisor. Elsevier.
- ↑ (April 2014) Personality traits and vulnerability or resilience to substance use disorders. Trends in Cognitive Sciences 18 (4): 211–7.
- ↑ (2019) Personality Traits and Drug Consumption. A Story Told by Data. Springer, Cham. DOI:10.1007/978-3-030-10442-9. ISBN 978-3-030-10441-2.
- ↑ 127.00 127.01 127.02 127.03 127.04 127.05 127.06 127.07 127.08 127.09 127.10 127.11 127.12 127.13 127.14 127.15 (2014). Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience 264: 198–206.
- ↑ Mayfield RD, Harris RA,1, Schuckit MA (May 2008) "Genetic factors influencing alcohol dependence" PMID 18362899
- ↑ 129.0 129.1 (May 1994) A twin-family study of alcoholism in women. Am J Psychiatry 151 (5): 707–15.
- ↑ (2013). Low frequency genetic variants in the μ-opioid receptor (OPRM1) affect risk for addiction to heroin and cocaine. Neuroscience Letters 542: 71–75.
- ↑ (December 2013) Implications of genome wide association studies for addiction: Are our a priori assumptions all wrong?. Pharmacology & Therapeutics 140 (3): 267–79.
- ↑ Genetics of alcoholism. Alcohol Health and Research World: 1–11.
- ↑ Life, Dr. Steven Melemis, I Want to Change My, "The Genetics of Addiction – Is Addiction a Disease?", I Want to Change My Life.
- ↑ Abuse, National Institute on Drug, "Overdose Death Rates", 9 August 2018.
- ↑ 135.0 135.1 Abuse, National Institute on Drug, "What are risk factors and protective factors?".
- ↑ 136.0 136.1 Adverse Childhood Experiences. Substance Abuse and Mental Health Services Administration. Retrieved 26 September 2016.
- ↑ 137.0 137.1 (2011). The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology 214 (1): 17–31.
- ↑ Environmental Risk Factors.
- ↑ (June 2000) The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews 24 (4): 417–63.
- ↑ (April 2014) Neurobiology of adolescent substance use and addictive behaviors: treatment implications. Adolescent Medicine 25 (1): 15–32.
- ↑ (1990). Evaluation of the effectiveness of adolescent drug abuse treatment, assessment of risks for relapse, and promising approaches for relapse prevention. The International Journal of the Addictions 25 (9A–10A): 1085–140.
- ↑ (November 2008) Practitioner review: adolescent alcohol use disorders: assessment and treatment issues. Journal of Child Psychology and Psychiatry, and Allied Disciplines 49 (11): 1131–54.
- ↑ Age and Substance Abuse – Alcohol Rehab.
- ↑ 144.0 144.1 144.2 Nationwide Trends. National Institute on Drug Abuse (June 2015). Retrieved 15 December 2017.
- ↑ 145.0 145.1 "Addiction Statistics – Facts on Drug and Alcohol Addiction", AddictionCenter.
- ↑ SAMHSA. Risk and Protective Factors. Substance Abuse and Mental Health Administration.
- ↑ Infographic – Risk Factors of Addiction | Recovery Research Institute.
- ↑ Drug addiction Risk factors – Mayo Clinic.
- ↑ "The Connection Between Mental Illness and Substance Abuse | Dual Diagnosis", Dual Diagnosis.
- ↑ 150.0 150.1 150.2 (2015). Transgenerational Inheritance of Paternal Neurobehavioral Phenotypes: Stress, Addiction, Ageing and Metabolism. Mol. Neurobiol. 53 (9): 6367–76.
- ↑ Chen CC, Yin SJ. (Oct 2008) "Alcohol abuse and related factors in Asia". PMID: 19012127
- ↑ Mak KK, Lai CM, Watanabe H. (Nov 2014) "Epidemiology of internet behaviors and addiction among adolescents in six Asian countries". PMID: 25405785
- ↑ The Mental Health of Australians 2: Substance Use Disorders in Australia. Department of Health and Ageing, Canberra (May 2009).
- ↑ Peacock A, Leung J, Larney S. (Oct 2018) "Global statistics on alcohol, tobacco and illicit drug use: 2017 status report." PMID: 29749059
- ↑ (March 2011) Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Primary Care 38 (1): 71–90.
- ↑ 156.0 156.1 156.2 156.3 Nora Volkow (31 March 2016). A Major Step Forward for Addiction Medicine. National Institutes of Health. Retrieved 3 April 2016.
- ↑ Gramlich J (26 October 2017). Nearly half of Americans have a family member or close friend who's been addicted to drugs. Pew Research Center. Retrieved 14 January 2018.
- ↑ "We were addicted to their pill, but they were addicted to the money", Washington Post. Retrieved 22 July 2019. (written in en)
- ↑ World Drug Report 2012 UN, 2012.
- ↑ Pacurucu Castillo, José Marcelo, Adrián Hernández, Renato D. Alarcón (2019) "World Opioid and Substance Use Epidemic: A Latin American Perspective" Psychiatryonline.org
- Image legend
ReferencesISBN links support NWE through referral fees
- Maia Szalavitz (2016). Unbroken Brain. St. Martin's Press. ISBN 978-1250055828.
External links
- The Science of Addiction: Genetics and the Brain. Learn.Genetics – University of Utah.
- Why do our brains get addicted? – a TEDMED 2014 talk by Nora Volkow, the director of the National Institute on Drug Abuse at NIH.
- Laterality of Brain Activation for Risk Factors of Addiction
Kyoto Encyclopedia of Genes and Genomes (KEGG) signal transduction pathways:
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
Cite error: <ref> tags exist for a group named "note", but no corresponding <references group="note"/> tag was found