Difference between revisions of "Barite" - New World Encyclopedia
| (13 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Copyedited}}{{Images OK}}{{Submitted}}{{Approved}}{{Paid}} |
[[Image:barite09.jpg|thumb|right|250px|Large barite crystals from Nevada.]] | [[Image:barite09.jpg|thumb|right|250px|Large barite crystals from Nevada.]] | ||
| − | |||
| − | |||
| − | |||
| − | '''Barite''' ([[Barium|Ba]][[Sulfur|S]][[Oxygen|O]]<sub>4</sub>) | + | '''Barite''' (British spelling '''Baryte'''), also called '''heavy spar''', is a [[mineral]] that is widely distributed around the globe. Chemically, it consists of [[crystal]]s of [[barium sulfate]] ([[Barium|Ba]][[Sulfur|S]][[Oxygen|O]]<sub>4</sub>). The pure crystals are white or [[color]]less, but the mineral acquires various colors based on impurities it contains. |
| + | {{toc}} | ||
| + | This mineral is the main source of [[barium]] and its [[chemical compound|compounds]]. When crushed, it is used as an aggregate in a "heavy" [[cement]], and the finely ground material is used as a filler in such products as [[paper]], [[textile]]s, [[plastic]]s, and [[rubber]]. In addition, it is an ingredient in [[paint]]s and in a type of [[drilling fluid|mud]] used for sealing [[petroleum]] wells during drilling. | ||
| + | [[Image:Baryte Morocco.jpg|thumb|right|Crystals of barite (pink) mixed with cerussite (clear) from Morocco.]] | ||
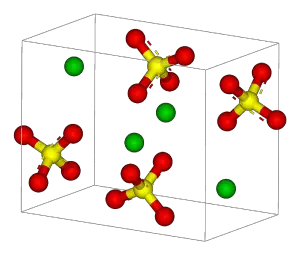
| + | [[Image:Barite-unit-cell-3D-balls.png|thumb|right|The unit cell in barite crystals.]] | ||
| + | [[Image:Baryte Poland.jpg|thumb|right|Barite (cream and brown bands) with galena (silvery) and hematite from Poland.]] | ||
| − | + | == Occurrence == | |
| − | + | Barite commonly occurs in lead-zinc veins in [[limestone]]s, in hot spring deposits, and with [[hematite]] ore. It is often associated with the [[mineral]]s [[anglesite]] and [[Celestine (mineral)|celestine]]. | |
| − | + | == Etymology and History == | |
| − | == | + | The name barite is derived from the [[Greek language|Greek]] word ''βαρύς'', meaning "heavy." The radiating form, sometimes referred to as Bologna Stone, attained some notoriety among [[Alchemy|alchemists]] for the [[phosphorescent]] specimens found in the 1600s near [[Bologna, Italy]] by [[Vincenzo Cascariolo]]. |
| + | |||
| + | == Characteristics == | ||
| + | |||
| + | Barite has an [[orthorhombic]] [[crystal]] structure. It is practically insoluble in [[water]] and in most other chemical reagents. It has a [[Mohs hardness]] of three. Its [[refractive index]] is 1.63, its [[specific gravity]] is in the range of 4.3-5. Under arid, sandy conditions, crystals of barite (or [[gypsum]]) may form a rosette structure embedded with sand grains, and this structure is colloquially referred to as a ''desert rose''. | ||
| + | |||
| + | == Production == | ||
| + | |||
| + | In [[commerce]], the mineral is sometimes referred to as "barytes." The first marketable product, called ''primary barite'', includes crude barite (run of mine) and the products of simple treatments such as washing, jigging, separation of heavy media, tabling, flotation, and magnetic separation. Most crude barite requires some upgrading to minimum purity or density. | ||
| + | |||
| + | == Uses == | ||
| − | + | Barite is the main source of barium and its compounds. It may be used as an aggregate in a "heavy" [[cement]], for which it is crushed and screened to a uniform size. Most barite is ground to a small, uniform size and then used as a filler in the production of such goods as paper, textiles, linoleum, rubber, and plastics. In addition, it is used in paints and in a special type of [[drilling fluid|mud]] for sealing [[petroleum]] wells in the course of drilling. | |
| − | + | == Precautions == | |
| − | + | Although barite contains barium, which is a "heavy" metal, it is not considered a [[toxic]] chemical by most governments because of its extreme [[solubility|insolubility]]. | |
| − | + | == References == | |
| − | * Mineral | + | * Farndon, John. 2006. ''The Practical Encyclopedia of Rocks & Minerals: How to Find, Identify, Collect and Maintain the World's best Specimens, with over 1000 Photographs and Artworks''. London: Lorenz Books. ISBN 0754815412 |
| + | * Klein, Cornelis, and Barbara Dutrow. 2007. ''Manual of Mineral Science''. 23rd ed. New York: John Wiley. ISBN 978-0471721574 | ||
| + | * Mineral Gallery. 2006. The Mineral Barite ''Amethyst Galleries''. | ||
| + | * Pellant, Chris. 2002. ''Rocks and Minerals''. Smithsonian Handbooks. New York: Dorling Kindersley. ISBN 0789491060 | ||
| + | * Shaffer, Paul R., Herbert S. Zim, and Raymond Perlman. 2001. ''Rocks, Gems and Minerals''. Rev. ed. New York: St. Martin's Press. ISBN 1582381321 | ||
==External links== | ==External links== | ||
| + | All links retrieved September 20, 2023. | ||
| − | * [http://www.mindat.org/min-549.html | + | * [http://minerals.usgs.gov/minerals/pubs/commodity/barite/ Barite: Statistics and Information] ''U.S. Geological Survey''. |
| − | * [http://webmineral.com/data/Barite.shtml | + | * [http://www.mindat.org/min-549.html Baryte] ''Mindat.org''. |
| − | + | * [http://webmineral.com/data/Barite.shtml Barite Mineral Data] ''Webmineral.com''. | |
| + | |||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Latest revision as of 08:06, 20 September 2023
Barite (British spelling Baryte), also called heavy spar, is a mineral that is widely distributed around the globe. Chemically, it consists of crystals of barium sulfate (BaSO4). The pure crystals are white or colorless, but the mineral acquires various colors based on impurities it contains.
This mineral is the main source of barium and its compounds. When crushed, it is used as an aggregate in a "heavy" cement, and the finely ground material is used as a filler in such products as paper, textiles, plastics, and rubber. In addition, it is an ingredient in paints and in a type of mud used for sealing petroleum wells during drilling.
Occurrence
Barite commonly occurs in lead-zinc veins in limestones, in hot spring deposits, and with hematite ore. It is often associated with the minerals anglesite and celestine.
Etymology and History
The name barite is derived from the Greek word βαρύς, meaning "heavy." The radiating form, sometimes referred to as Bologna Stone, attained some notoriety among alchemists for the phosphorescent specimens found in the 1600s near Bologna, Italy by Vincenzo Cascariolo.
Characteristics
Barite has an orthorhombic crystal structure. It is practically insoluble in water and in most other chemical reagents. It has a Mohs hardness of three. Its refractive index is 1.63, its specific gravity is in the range of 4.3-5. Under arid, sandy conditions, crystals of barite (or gypsum) may form a rosette structure embedded with sand grains, and this structure is colloquially referred to as a desert rose.
Production
In commerce, the mineral is sometimes referred to as "barytes." The first marketable product, called primary barite, includes crude barite (run of mine) and the products of simple treatments such as washing, jigging, separation of heavy media, tabling, flotation, and magnetic separation. Most crude barite requires some upgrading to minimum purity or density.
Uses
Barite is the main source of barium and its compounds. It may be used as an aggregate in a "heavy" cement, for which it is crushed and screened to a uniform size. Most barite is ground to a small, uniform size and then used as a filler in the production of such goods as paper, textiles, linoleum, rubber, and plastics. In addition, it is used in paints and in a special type of mud for sealing petroleum wells in the course of drilling.
Precautions
Although barite contains barium, which is a "heavy" metal, it is not considered a toxic chemical by most governments because of its extreme insolubility.
ReferencesISBN links support NWE through referral fees
- Farndon, John. 2006. The Practical Encyclopedia of Rocks & Minerals: How to Find, Identify, Collect and Maintain the World's best Specimens, with over 1000 Photographs and Artworks. London: Lorenz Books. ISBN 0754815412
- Klein, Cornelis, and Barbara Dutrow. 2007. Manual of Mineral Science. 23rd ed. New York: John Wiley. ISBN 978-0471721574
- Mineral Gallery. 2006. The Mineral Barite Amethyst Galleries.
- Pellant, Chris. 2002. Rocks and Minerals. Smithsonian Handbooks. New York: Dorling Kindersley. ISBN 0789491060
- Shaffer, Paul R., Herbert S. Zim, and Raymond Perlman. 2001. Rocks, Gems and Minerals. Rev. ed. New York: St. Martin's Press. ISBN 1582381321
External links
All links retrieved September 20, 2023.
- Barite: Statistics and Information U.S. Geological Survey.
- Baryte Mindat.org.
- Barite Mineral Data Webmineral.com.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.