Difference between revisions of "Sphalerite" - New World Encyclopedia
| Line 4: | Line 4: | ||
[[Image:Sphalerite-unit-cell-3D-balls.png|thumb|The unit cell of sphalerite]] | [[Image:Sphalerite-unit-cell-3D-balls.png|thumb|The unit cell of sphalerite]] | ||
| − | '''Sphalerite''' is a [[mineral]] that consists largely of [[zinc sulfide]] (ZnS) in [[crystal]]line form, but it almost always contains variable amounts of [[iron]]. It is the chief [[ore]] of [[zinc]]. When iron | + | '''Sphalerite''' is a [[mineral]] that consists largely of [[zinc sulfide]] (ZnS) in [[crystal]]line form, but it almost always contains variable amounts of [[iron]]. It is the chief [[ore]] of [[zinc]]. Its color is usually yellow, brown, or gray to gray-black, and it may be shiny or dull. When the content of iron is high, it is an opaque black variety known as '''marmatite''' ((Zn,Fe)S). |
| − | + | It is usually found in association with [[galena]], [[pyrite]], and other [[sulfide]]s, along with [[calcite]], [[dolomite]], and [[fluorite]]. Miners have referred to sphalerite as ''zinc blende'', ''mock lead'', ''false galena'', and ''black-jack''. | |
| − | + | == Properties == | |
| + | |||
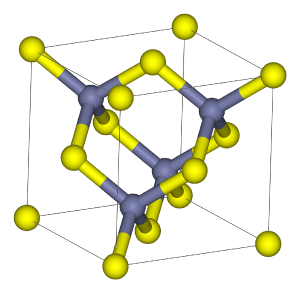
| + | The mineral crystallizes in the [[cubic (crystal system)|cubic]] [[crystal]] system. In the crystal structure, zinc and sulfur atoms are tetrahedrally coordinated. The structure is closely related to that of [[diamond]]. The [[hexagonal (crystal system)|hexagonal]] analog is known as the [[wurtzite]] structure. | ||
| + | |||
| + | Its [[luster]] is resinous. It has a yellow or light brown [[Mineral#Streak|streak]], a [[Mohs hardness scale|hardness]] of 3.5 - 4 on the Mohs scale, and a [[specific gravity]] of 3.9-4.1. Some specimens have a red [[iridescence]] within the gray-black crystals; these are called "ruby sphalerite." The pale yellow and red varieties have very little iron and are translucent. The darker, more opaque varieties contain more iron. Some specimens are also [[fluorescence|fluorescent]] in [[ultraviolet]] light. The [[refractive index]] of sphalerite (as measured via [[sodium light]] at 589.3 nanometers) is 2.37. | ||
| + | |||
| + | Sphalerite crystallizes in the [[Cubic (crystal system)|isometric]] crystal system and possesses perfect dodecahedral [[Cleavage (crystal)|cleavage]]. Gemmy, pale specimens from [[Franklin, New Jersey]] are highly fluorescent orange or blue under longwave ultraviolet light and are known as ''cleiophane'', an almost pure variety of ZnS. | ||
| + | |||
| + | == Uses == | ||
Crystals of suitable size and transparency have been fashioned into [[gemstone]]s, usually featuring the [[brilliant (diamond cut)|brilliant cut]] to best display sphalerite's high [[dispersion (optics)|dispersion]] of 0.156 (B-G interval)—over three times that of [[diamond]]. Freshly cut gems are lively with an adamantine luster and could conceivably be mistaken for a fancy-colored diamond in passing, but due to sphalerite's softness and fragility the gems are best left unset as collector's or [[museum]] pieces (although some have been set into pendants). Collectors may pay a premium for stones over one [[carat (mass)|carat]] (200 mg), as clean crystals are usually quite small. Gem-quality material is usually a yellowish to honey brown, red to orange, or green; the two most important sources are the Chivera mine, [[Cananea]], [[Sonora]], [[Mexico]]; and the [[Picos de Europa]], [[Cordillera Cantabrica]], near [[Santander, Cantabria|Santander]] on [[Spain]]'s northern coast. | Crystals of suitable size and transparency have been fashioned into [[gemstone]]s, usually featuring the [[brilliant (diamond cut)|brilliant cut]] to best display sphalerite's high [[dispersion (optics)|dispersion]] of 0.156 (B-G interval)—over three times that of [[diamond]]. Freshly cut gems are lively with an adamantine luster and could conceivably be mistaken for a fancy-colored diamond in passing, but due to sphalerite's softness and fragility the gems are best left unset as collector's or [[museum]] pieces (although some have been set into pendants). Collectors may pay a premium for stones over one [[carat (mass)|carat]] (200 mg), as clean crystals are usually quite small. Gem-quality material is usually a yellowish to honey brown, red to orange, or green; the two most important sources are the Chivera mine, [[Cananea]], [[Sonora]], [[Mexico]]; and the [[Picos de Europa]], [[Cordillera Cantabrica]], near [[Santander, Cantabria|Santander]] on [[Spain]]'s northern coast. | ||
Revision as of 19:41, 21 April 2007
Sphalerite is a mineral that consists largely of zinc sulfide (ZnS) in crystalline form, but it almost always contains variable amounts of iron. It is the chief ore of zinc. Its color is usually yellow, brown, or gray to gray-black, and it may be shiny or dull. When the content of iron is high, it is an opaque black variety known as marmatite ((Zn,Fe)S).
It is usually found in association with galena, pyrite, and other sulfides, along with calcite, dolomite, and fluorite. Miners have referred to sphalerite as zinc blende, mock lead, false galena, and black-jack.
Properties
The mineral crystallizes in the cubic crystal system. In the crystal structure, zinc and sulfur atoms are tetrahedrally coordinated. The structure is closely related to that of diamond. The hexagonal analog is known as the wurtzite structure.
Its luster is resinous. It has a yellow or light brown streak, a hardness of 3.5 - 4 on the Mohs scale, and a specific gravity of 3.9-4.1. Some specimens have a red iridescence within the gray-black crystals; these are called "ruby sphalerite." The pale yellow and red varieties have very little iron and are translucent. The darker, more opaque varieties contain more iron. Some specimens are also fluorescent in ultraviolet light. The refractive index of sphalerite (as measured via sodium light at 589.3 nanometers) is 2.37.
Sphalerite crystallizes in the isometric crystal system and possesses perfect dodecahedral cleavage. Gemmy, pale specimens from Franklin, New Jersey are highly fluorescent orange or blue under longwave ultraviolet light and are known as cleiophane, an almost pure variety of ZnS.
Uses
Crystals of suitable size and transparency have been fashioned into gemstones, usually featuring the brilliant cut to best display sphalerite's high dispersion of 0.156 (B-G interval)—over three times that of diamond. Freshly cut gems are lively with an adamantine luster and could conceivably be mistaken for a fancy-colored diamond in passing, but due to sphalerite's softness and fragility the gems are best left unset as collector's or museum pieces (although some have been set into pendants). Collectors may pay a premium for stones over one carat (200 mg), as clean crystals are usually quite small. Gem-quality material is usually a yellowish to honey brown, red to orange, or green; the two most important sources are the Chivera mine, Cananea, Sonora, Mexico; and the Picos de Europa, Cordillera Cantabrica, near Santander on Spain's northern coast.
See also
ReferencesISBN links support NWE through referral fees
- Farndon, John. 2006. The Practical Encyclopedia of Rocks & Minerals: How to Find, Identify, Collect and Maintain the World's best Specimens, with over 1000 Photographs and Artworks. London: Lorenz Books. ISBN 0754815412.
- Klein, Cornelis, and Barbara Dutrow. 2007. Manual of Mineral Science. 23rd ed. New York: John Wiley. ISBN 978-0471721574.
- Pellant, Chris. 2002. Rocks and Minerals. Smithsonian Handbooks. New York: Dorling Kindersley. ISBN 0789491060.
- Shaffer, Paul R., Herbert S. Zim, and Raymond Perlman. 2001. Rocks, Gems and Minerals. Rev. ed. New York: St. Martin's Press. ISBN 1582381321.
- Mindat.org. 2007. Sphalerite. Mindat.org. Retrieved April 10, 2007.
- Mineral Gallery. 2006. The Mineral Sphalerite. Amethyst Galleries. Retrieved April 10, 2007.
External links
- Sphalerite - Minerals.net. Retrieved April 10, 2007.
- Sphalerite - The Minerals of Franklin and Sterling Hill, New Jersey. Retrieved April 10, 2007.
- The Zincblende (B3) Structure. Retrieved April 10, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.