Difference between revisions of "Salicylic acid" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 49: | Line 49: | ||
In salicylic acid the OH group is adjacent to the carboxyl group. | In salicylic acid the OH group is adjacent to the carboxyl group. | ||
| + | It is only slightly soluable in water, but is soluble in ethanol and ether. | ||
==Production== | ==Production== | ||
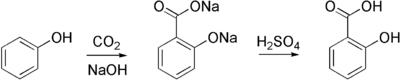
| − | + | Salicylic acid is commercially prepared sodium salicylate, which is produced from sodium [[phenol|phenoxide]] and [[carbon dioxide]] at high pressure and temperature in the Kolbe-Schmitt reaction. Sodium salicylate is acidified to give the desired salicylic acid: | |
:[[Image:Kolbe-Schmitt.png|left|400px]]{{clear}} | :[[Image:Kolbe-Schmitt.png|left|400px]]{{clear}} | ||
| + | To produce aspirin, salicylic acid is then acetylated using acetic anhydride, yielding aspirin and acetic acid as a byproduct. By using a process involving the esterification of the phenolic hydroxyl group of salicylic acid, it retains some of its potency as an analgesic while reducing its acidity. | ||
| − | + | ==Uses== | |
| − | + | ===Aspirin=== | |
| + | [[Image:Aspirin.jpg|thumb|right|Aspirin (acetylsalicylic acid)]] | ||
| + | One of the key uses of salicylic acid is for the production of aspirin. Aspirin or acetylsalicylic acid is a [[drug]] often used as an ''analgesic'' (against minor pains and aches), ''antipyretic'' (against [[fever]]), and ''anti-inflammatory'' (against localized redness, swelling, heat, and pain). It has also an anticoagulant ("blood-thinning") effect and is used in long-term low-doses to prevent [[heart attack]]s. | ||
| + | The medicinal properties of salicylate (mainly for [[fever]] relief) have been known since ancient times. The substance occurs in the bark of [[willow]] trees; the name ''salicylic acid'' is derived from ''salix'', the [[Latin]] name for the willow tree (Mackowiak 2000). [[Hippocrates]], a [[Greece|Greek]] physician, wrote in the fifth century <small>B.C.E.</small> about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers. This remedy is also mentioned in texts from ancient [[Sumeria]], [[Egypt]], and [[Assyria]]. Native Americans claim to have used it for headaches, fever, sore muscles, rheumatism, and chills. The Reverend Edward Stone, a vicar from Chipping Norton, Oxfordshire, England, noted in 1763 that the bark of the willow was effective in reducing a fever.[http://www.gud90.dial.pipex.com/Stone8.html] | ||
| + | The active extract of the bark, called ''salicin'', after the [[Latin]] name for the White willow (''Salix alba''), was isolated to its crystalline form in 1828 by Henri Leroux, a [[France|French]] pharmacist, and Raffaele Piria, an [[Italy|Italian]] chemist, who then succeeded in separating out the acid in its pure state. Salicin is highly acidic when in a saturated solution with water ([[pH]] = 2.4), and is called salicylic acid for that reason. | ||
| + | This chemical was also isolated from meadowsweet flowers (genus ''Filipendula'', formerly classified in ''Spiraea'') by German researchers in 1839. | ||
| + | ===Other uses=== | ||
| + | [[Image:Salicylic acid pads.jpg|thumb|left|[[Cotton]] pads soaked in salicylic acid can be used to chemically exfoliate skin]] | ||
| + | Also known as 2-hydroxybenzoic acid (one of several beta hydroxy acids), salicylic acid is the key additive in many skin-care products for the treatment of [[acne]], [[psoriasis]], [[callus|calluses]], corns, and keratosis pilaris. It treats acne by causing skin cells to slough off more readily, preventing pores from clogging up. This effect on skin cells also makes salicylic acid an active ingredient in several [[shampoo]]s meant to treat [[dandruff]]. Salicylic acid is also used as an active ingredient in gels which remove verrucas (plantar warts). | ||
| − | + | Use of straight salicylic solution may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock (Grimes 1999, Roberts 2004). | |
| − | |||
| − | + | Subsalicylate in combination with [[bismuth]] form the popular stomach relief aid known commonly as Pepto-Bismol. When combined, the two key ingredients help control [[diarrhea]], nausea, heartburn, and gas. It is also a very mild [[antibiotic]]. | |
| − | |||
| − | |||
| − | + | Toxicological effects of 100% salicylic acid, however, are mostly harmful. It is harmful by ingestion, inhalation, and through skin absorption. It acts as an irritant, and chronic effects have shown 100% salicylic acid to cause [[DNA]] damage, and also cause [[allergy|allergic]] reactions after repeated exposure. This is why most acne treatment medications use a percent range of 2-5 in solution. | |
| − | |||
| − | [[ | ||
| − | + | While salicylic acid is toxic if ingested in large quantities, in small quantities is used as a [[food preservative]] and [[antiseptic]] in [[toothpaste]]. For some people with salicylate sensitivity, even these small doses can be harmful. | |
| − | + | Various salts of salicylic acid (methyl salicylate, phenyl salicylate, salicylanilide) are used for flavorings, sunburn creams, pill coatings, and skin fungicide, and salicylic acid is used in producing dyes as well. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== See also == | == See also == | ||
| − | |||
| − | |||
* [[Phenol]] | * [[Phenol]] | ||
* [[Aspirin]] | * [[Aspirin]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Grimes, P. E. 1999. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. ''Dermatologic Surgery'' 25: 18-22. | |
| − | + | * Mackowiak, P. A. 2000. Brief history of antipyretic therapy. ''Clinical Infectious Diseases'' 31: 154–156. | |
| − | + | * Roberts, W. E. 2004. Chemical peeling in ethnic/dark skin. ''Dermatologic Therapy'' 17(2): 196. | |
| − | |||
[[Category:Life sciences]] | [[Category:Life sciences]] | ||
{{credit|120193336}} | {{credit|120193336}} | ||
Revision as of 00:58, 9 April 2007
| Salicylic acid | |
|---|---|
| |
| Chemical name | 2-Hydroxybenzoic acid |
| Chemical formula | C7H6O3 |
| Molecular mass | 138.123 g/mol |
| Melting point | 160 °C |
| Boiling point | 211 °C (2666 Pa) |
| Density | 1.44 g/cm³ (at 20 °C) |
| pKa | 2.97 |
| CAS number | [69-72-7] |
| SMILES | c1(O)ccccc1C(O)=O |
| Disclaimer and references | |
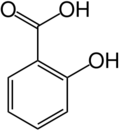
Salicylic acid is a crystalline, solid (up to 159oC) organic acid that is used to make aspirin and various pharmaceutical products, including for skin conditions. It also functions as a plant hormone. The name derives from the Latin word for the willow tree (Salix), from whose bark it can be obtained.
Chemistry
The chemical formula for salicyclic acid is C6H4(OH)CO2H.
Salicylic acid is both a caroxylic acid and a phenol. A carboxylic acid is an organic (carbon-containing) acid characterized by the presence of a carboxyl group, which has the formula -C(=O)OH, usually written -COOH or -CO2H. A phenol, in the general sense of the term, is any any compound which contains a six-membered aromatic ring, bonded directly to a hydroxyl group (-OH).
In salicylic acid the OH group is adjacent to the carboxyl group.
It is only slightly soluable in water, but is soluble in ethanol and ether.
Production
Salicylic acid is commercially prepared sodium salicylate, which is produced from sodium phenoxide and carbon dioxide at high pressure and temperature in the Kolbe-Schmitt reaction. Sodium salicylate is acidified to give the desired salicylic acid:
To produce aspirin, salicylic acid is then acetylated using acetic anhydride, yielding aspirin and acetic acid as a byproduct. By using a process involving the esterification of the phenolic hydroxyl group of salicylic acid, it retains some of its potency as an analgesic while reducing its acidity.
Uses
Aspirin
One of the key uses of salicylic acid is for the production of aspirin. Aspirin or acetylsalicylic acid is a drug often used as an analgesic (against minor pains and aches), antipyretic (against fever), and anti-inflammatory (against localized redness, swelling, heat, and pain). It has also an anticoagulant ("blood-thinning") effect and is used in long-term low-doses to prevent heart attacks.
The medicinal properties of salicylate (mainly for fever relief) have been known since ancient times. The substance occurs in the bark of willow trees; the name salicylic acid is derived from salix, the Latin name for the willow tree (Mackowiak 2000). Hippocrates, a Greek physician, wrote in the fifth century B.C.E. about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers. This remedy is also mentioned in texts from ancient Sumeria, Egypt, and Assyria. Native Americans claim to have used it for headaches, fever, sore muscles, rheumatism, and chills. The Reverend Edward Stone, a vicar from Chipping Norton, Oxfordshire, England, noted in 1763 that the bark of the willow was effective in reducing a fever.[1]
The active extract of the bark, called salicin, after the Latin name for the White willow (Salix alba), was isolated to its crystalline form in 1828 by Henri Leroux, a French pharmacist, and Raffaele Piria, an Italian chemist, who then succeeded in separating out the acid in its pure state. Salicin is highly acidic when in a saturated solution with water (pH = 2.4), and is called salicylic acid for that reason. This chemical was also isolated from meadowsweet flowers (genus Filipendula, formerly classified in Spiraea) by German researchers in 1839.
Other uses

Also known as 2-hydroxybenzoic acid (one of several beta hydroxy acids), salicylic acid is the key additive in many skin-care products for the treatment of acne, psoriasis, calluses, corns, and keratosis pilaris. It treats acne by causing skin cells to slough off more readily, preventing pores from clogging up. This effect on skin cells also makes salicylic acid an active ingredient in several shampoos meant to treat dandruff. Salicylic acid is also used as an active ingredient in gels which remove verrucas (plantar warts).
Use of straight salicylic solution may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock (Grimes 1999, Roberts 2004).
Subsalicylate in combination with bismuth form the popular stomach relief aid known commonly as Pepto-Bismol. When combined, the two key ingredients help control diarrhea, nausea, heartburn, and gas. It is also a very mild antibiotic.
Toxicological effects of 100% salicylic acid, however, are mostly harmful. It is harmful by ingestion, inhalation, and through skin absorption. It acts as an irritant, and chronic effects have shown 100% salicylic acid to cause DNA damage, and also cause allergic reactions after repeated exposure. This is why most acne treatment medications use a percent range of 2-5 in solution.
While salicylic acid is toxic if ingested in large quantities, in small quantities is used as a food preservative and antiseptic in toothpaste. For some people with salicylate sensitivity, even these small doses can be harmful.
Various salts of salicylic acid (methyl salicylate, phenyl salicylate, salicylanilide) are used for flavorings, sunburn creams, pill coatings, and skin fungicide, and salicylic acid is used in producing dyes as well.
See also
ReferencesISBN links support NWE through referral fees
- Grimes, P. E. 1999. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatologic Surgery 25: 18-22.
- Mackowiak, P. A. 2000. Brief history of antipyretic therapy. Clinical Infectious Diseases 31: 154–156.
- Roberts, W. E. 2004. Chemical peeling in ethnic/dark skin. Dermatologic Therapy 17(2): 196.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.