Difference between revisions of "Nanoparticle" - New World Encyclopedia
m |
m |
||
| Line 33: | Line 33: | ||
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk [[copper]] (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same [[malleability]] and [[ductility]] as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their magnetisation direction using room temperature thermal energy, thus making them useless for memory storage. [[suspension (chemistry)|Suspension]]s of nanoparticles are possible because the interaction of the particle surface with the [[solvent]] is strong enough to overcome differences in [[density]], which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visible properties because they are small enough to confine their electrons and produce quantum effects. For example [[gold]] nanoparticles appear deep red to black in solution. | Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk [[copper]] (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same [[malleability]] and [[ductility]] as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their magnetisation direction using room temperature thermal energy, thus making them useless for memory storage. [[suspension (chemistry)|Suspension]]s of nanoparticles are possible because the interaction of the particle surface with the [[solvent]] is strong enough to overcome differences in [[density]], which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visible properties because they are small enough to confine their electrons and produce quantum effects. For example [[gold]] nanoparticles appear deep red to black in solution. | ||
| − | Nanoparticles have a very high surface area to volume ratio. This provides a tremendous driving force for [[diffusion]], especially at elevated temperatures. [[Sintering]] can take place at lower temperatures, over shorter time scales than for larger particles. This theoretically does not affect the [[density]] of the final product, though flow difficulties and the tendency of nanoparticles to agglomerate complicates matters. The large surface area to volume ratio also reduces the incipient [[melting point depression|melting temperature]] of nanoparticles <ref>Buffat, P.H., and J.P. Burrel. 1976. [http://link.aps.org/abstract/PRA/v13/p2287 Size effect on the melting temperature of gold particles.] ''Physical Review A.'' 13(6):2287–2298.</ref>. | + | Nanoparticles have a very high surface area to volume ratio. This provides a tremendous driving force for [[diffusion]], especially at elevated temperatures. [[Sintering]] can take place at lower temperatures, over shorter time scales than for larger particles. This theoretically does not affect the [[density]] of the final product, though flow difficulties and the tendency of nanoparticles to agglomerate complicates matters. The large surface area to volume ratio also reduces the incipient [[melting point depression|melting temperature]] of nanoparticles <ref>Buffat, P.H., and J.P. Burrel. 1976. [http://link.aps.org/abstract/PRA/v13/p2287 Size effect on the melting temperature of gold particles.] ''Physical Review A.'' 13(6):2287–2298. Retrieved November 16, 2008.</ref>. |
Moreover nanoparticles have been found to impart some extra properties to various day to day products. Like the presence of titanium dioxide nanoparticles impart what we call as the self-cleaning effect, and the size being nanorange, the particles can't be seen. Nano Zinc Oxide particles have been found to have superior UV blocking properties compared to its bulk substitute. This is one of the reasons why it is often used in the sunscreen lotions. | Moreover nanoparticles have been found to impart some extra properties to various day to day products. Like the presence of titanium dioxide nanoparticles impart what we call as the self-cleaning effect, and the size being nanorange, the particles can't be seen. Nano Zinc Oxide particles have been found to have superior UV blocking properties compared to its bulk substitute. This is one of the reasons why it is often used in the sunscreen lotions. | ||
Revision as of 00:39, 16 November 2008
|
Part of a series of articles on |
|
Fullerenes |
|
Nanoparticles |
|
See also |
In nanotechnology, a particle is defined as a small object that behaves as a whole unit in terms of its transport and properties. It is further classified according to size: In terms of diameter, fine particles cover a range between 100 and 2500 nanometers, while ultrafine particles, on the other hand, are sized between 1 and 100 nanometers. Similarly to ultrafine particles, nanoparticles are sized between 1 and 100 nanometers, though the size limitation can be restricted to two dimensions. Nanoparticles may or may not exhibit size-related intensive properties that differ significantly from those observed in fine particles or bulk materials.[1]
Nanoclusters[2] have at least one dimension between 1 and 10 nanometers and a narrow size distribution. Nanopowders[2] are agglomerates of ultrafine particles, nanoparticles, or nanoclusters. Nanometer sized single crystals, or single-domain ultrafine particles, are often referred to as nanocrystals. The term NanoCrystal® is a registered trademark[3] of Elan Pharma International (EPIL) used in relation to EPIL’s proprietary milling process and nanoparticulate drug formulations.
Nanoparticle research is currently an area of intense scientific research, due to a wide variety of potential applications in biomedical, optical, and electronic fields. The National Nanotechnology Initiative has led to generous public funding for nanoparticle research in the United States.
History
Although generally nanoparticles are considered an invention of modern science, they actually have a very long history. Specifically, nanoparticles were used by artisans as far back as in the 9th century Mesopotamia for generating a glittering effect on the surface of pot.
Even these days pottery from the Middle Ages and Renaissance often retain a distinct gold or copper colored metallic glitter. This so called lustre is caused by a metallic film that was applied to the transparent surface of a glazing. The lustre can still be visible if the film has resisted atmospheric oxidation and other weathering.
The lustre originates within the film itself, which contains silver and copper nanoparticles, dispersed homogeneously in the glassy matrix of the ceramic glaze. These nanoparticles were created by the artisans by adding copper and silver salts and oxides together with vinegar, ochre, and clay, on the surface of previously-glazed pottery. The object was then placed to a kiln and heated to about 600°C in a reducing atmosphere.
In the heat the glaze would soften, causing the copper and silver ions to migrate into the outer layers of the glaze. There the reducing atmosphere reduced the ions back to metals, which then came together forming the nanoparticles that give the colour and optical effects.
Lustre technique shows that craftsmen had a rather sophisticated empirical knowledge of materials. The technique originates in the islamic world. As Muslims were not allowed to use gold in artistic representations, they had to find a way to create a similar effect without using real gold. The solution they found was using lustre.
Michael Faraday provided the first description, in scientific terms, of the optical properties of nanometer-scale metals in his classic 1857 paper "Experimental relations of gold (and other metals) to light."[4]
Much of the modern day studies of these objects have been conducted at the ESRF laboratory. Several techniques were used to characterise the chemical and physical properties of these lustre, such as Rutherford Backscattering Spectrometry (RBS), optical absorption in the visible-ultraviolet region, electron microscopy (TEM and SEM),
Properties
Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and atomic or molecular structures. A bulk material should have constant physical properties regardless of its size, but at the nano-scale this is often not the case. Size-dependent properties are observed such as quantum confinement in semiconductor particles, surface plasmon resonance in some metal particles and superparamagnetism in magnetic materials.
The properties of materials change as their size approaches the nanoscale and as the percentage of atoms at the surface of a material becomes significant. For bulk materials larger than one micrometre the percentage of atoms at the surface is minuscule relative to the total number of atoms of the material. The interesting and sometimes unexpected properties of nanoparticles are partly due to the aspects of the surface of the material dominating the properties in lieu of the bulk properties.
Nanoparticles exhibit a number of special properties relative to bulk material. For example, the bending of bulk copper (wire, ribbon, etc.) occurs with movement of copper atoms/clusters at about the 50 nm scale. Copper nanoparticles smaller than 50 nm are considered super hard materials that do not exhibit the same malleability and ductility as bulk copper. The change in properties is not always desirable. Ferroelectric materials smaller than 10 nm can switch their magnetisation direction using room temperature thermal energy, thus making them useless for memory storage. Suspensions of nanoparticles are possible because the interaction of the particle surface with the solvent is strong enough to overcome differences in density, which usually result in a material either sinking or floating in a liquid. Nanoparticles often have unexpected visible properties because they are small enough to confine their electrons and produce quantum effects. For example gold nanoparticles appear deep red to black in solution.
Nanoparticles have a very high surface area to volume ratio. This provides a tremendous driving force for diffusion, especially at elevated temperatures. Sintering can take place at lower temperatures, over shorter time scales than for larger particles. This theoretically does not affect the density of the final product, though flow difficulties and the tendency of nanoparticles to agglomerate complicates matters. The large surface area to volume ratio also reduces the incipient melting temperature of nanoparticles [5].
Moreover nanoparticles have been found to impart some extra properties to various day to day products. Like the presence of titanium dioxide nanoparticles impart what we call as the self-cleaning effect, and the size being nanorange, the particles can't be seen. Nano Zinc Oxide particles have been found to have superior UV blocking properties compared to its bulk substitute. This is one of the reasons why it is often used in the sunscreen lotions. Clay nanoparticles when incorporated into polymer matrices increase re-inforcement, leading to stronger plastics, verified by a higher glass transition temperature and other mechanical property tests. These nanoparticles are hard, and impart their properties to the polymer (plastic). Nanoparticles have also been attached to textile fibers in order to create smart and functional clothing.
Classification
At the small end of the size range, nanoparticles are often referred to as clusters. Nanospheres, nanorods, and nanocups are just a few of the shapes that have been grown.
Metal, dielectric, and semiconductor nanoparticles have been formed, as well as hybrid structures (e.g., core-shell nanoparticles). Nanoparticles made of semiconducting material may also be labeled quantum dots if they are small enough (typically sub 10 nm) that quantization of electronic energy levels occurs. Such nanoscale particles are used in biomedical applications as drug carriers or imaging agents.
Semi-solid and soft nanoparticles have been manufactured. A prototype nanoparticle of semi-solid nature is the liposome. Various types of liposome nanoparticles are currently used clinically as delivery systems for anticancer drugs and vaccines.
Characterization
Nanoparticle characterization is necessary to establish understanding and control of nanoparticle synthesis and applications. Characterization is done by using a variety of different techniques, mainly drawn from materials science. Common techniques are electron microscopy [TEM,SEM], atomic force microscopy [AFM], dynamic light scattering [DLS], x-ray photoelectron spectroscopy [XPS], powder x-ray diffractometry [XRD], Fourier transform infrared spectroscopy [FTIR], Matrix-Assisted Laser-Desorption Time-of-flight mass spectrometry [MALDI-TOF], and Ultraviolet-visible spectroscopy.
Whilst the theory has been known for over a century (see Robert Brown), the technology for Nanoparticle tracking analysis (NTA) allows direct tracking of the Brownian motion and this method therefore allows the sizing of individual nanoparticles in solution.
Fabrication of nanoparticles
There are several methods for creating nanoparticles; attrition and pyrolysis are common methods. In attrition, macro or micro scale particles are ground in a ball mill, a planetary ball mill, or other size reducing mechanism. The resulting particles are air classified to recover nanoparticles.
In pyrolysis, a vaporous precursor (liquid or gas) is forced through an orifice at high pressure and burned. The resulting solid (a version of soot) is air classified to recover oxide particles from by-product gases. Pyrolysis often results in aggregates and agglomerates rather than singleton primary particles.
A thermal plasma can also deliver the energy necessary to cause evaporation of small micrometre size particles. The thermal plasma temperatures are in the order of 10000 K, so that solid powder easily evaporates. Nanoparticles are formed upon cooling while exiting the plasma region. The main types of the thermal plasmas torches used to produce nanoparticles are dc plasma jet, dc arc plasma and radio frequency (RF) induction plasmas. In the arc plasma reactors, the energy necessary for evaporation and reaction is provided by an electric arc which forms between the anode and the cathode. For example, silica sand can be vaporized with an arc plasma at atmospheric pressure. The resulting mixture of plasma gas and silica vapour can be rapidly cooled by quenching with oxygen, thus ensuring the quality of the fumed silica produced. In RF induction plasma torches, energy coupling to the plasma is accomplished through the electromagnetic field generated by the induction coil. The plasma gas does not come in contact with electrodes, thus eliminating possible sources of contamination and allowing the operation of such plasma torches with a wide range of gases including inert, reducing, oxidizing and other corrosive atmospheres. The working frequency is typically between 200 kHz and 40 MHz. Laboratory units run at power levels in the order of 30-50 kW while the large scale industrial units have been tested at power levels up to 1 MW. As the residence time of the injected feed droplets in the plasma is very short it is important that the droplet sizes are small enough in order to obtain complete evaporation. The RF plasma method has been used to synthesize different nanoparticle materials, for example synthesis of various ceramic nanoparticles such as oxides, carbours/carbides and nitrides of Ti and Si.
Inert-gas aggregation is frequently used to make nanoparticles from metals with low melting points. The metal is vaporized in a vacuum chamber and then supercooled with an inert gas stream. The supercooled metal vapor condenses in to nanometer-sized particles, which can be entrained in the inert gas stream and deposited on a substrate or studied in situ.
Nanoparticle Morphology
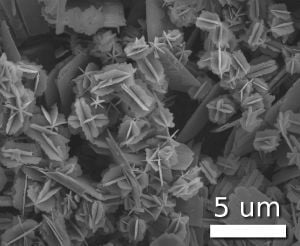
Scientists have taken to naming their particles after the real world shapes that they might represent. Nanospheres[6], nanoreefs[7], nanoboxes[8] and more have appeared in the literature. These morphologies sometimes arise spontaneously as an effect of a templating or directing agent present in the synthesis such as micellular emulsions or anodized alumina pores, or from the innate crystallographic growth patterns of the materials themselves.[9] Some of these morphologies may serve a purpose, such as long carbon nanotubes being used to bridge an electrical junction, or just a scientific curiosity like the stars shown at left.
Safety Issues
Nanoparticles present possible dangers, both medically and environmentally.[10] Most of these are due to the high surface to volume ratio, which can make the particles very reactive or catalytic.[11] They are also able to pass through cell membranes in organisms, and their interactions with biological systems are relatively unknown.[12] However, free nanoparticles in the environment quickly tend to agglomerate and thus leave the nano-regime, and nature itself presents many nanoparticles to which organisms on earth may have evolved immunity (such as salt particulates from ocean aerosols, terpenes from plants, or dust from volcanic eruptions)[citation needed]. A fuller analysis is provided in the article on nanotechnology.
According to the San Francisco Chronicle, "Animal studies have shown that some nanoparticles can penetrate cells and tissues, move through the body and brain and cause biochemical damage they also have shown to cause a risk factor in men for testicular cancer. But whether cosmetics and sunscreens containing nanomaterials pose health risks remains largely unknown, pending completion of long-range studies recently begun by the FDA and other agencies."[13] Diesel nanoparticles have been found to damage the cardiovascular system in a mouse model.[14]
Silicon nanoparticle cell
Generally, solar cells on the market today do not produce much electricity from ultraviolet light, instead it is either filtered out or absorbed by the cell, heating the cell. That heat is wasted energy and could even lead to damage to the cell. By diluting particles of silicon in alcohol, covering a solar cell with it and letting the alcohol evaporate to leave the nanoparticles of silicon on the cell has been increased the cell power output by 67% in the ultraviolet range and about 10% in the visible range [15].
See also
- Gallium selenide
- Indium selenide
- Liposome
- Magnetic nanoparticles
- Magnetic immunoassay
- Nanobiotechnology
- Nanocrystalline silicon
- Nanomaterials
- Nanoparticle Tracking Analysis
- Nanotechnology
- Photonic crystal
- Plasmon
- Quantum dot
- Silver Nano
- Silicon
Notes
- ↑ ASTM E 2456 - 06 Standard Terminology Relating to Nanotechnology. ASTM. Retrieved November 15, 2008.
- ↑ 2.0 2.1 Fahlman, B.D. 2007. Materials Chemistry. Dordrecht, NL: Springer. ISBN 9781402061196. pages 282-283.
- ↑ See for example US TM Reg. Nos. 2386089 / 2492925 and EU CTM Reg. No. 000885079
- ↑ Faraday, Michael. 1857. Experimental relations of gold (and other metals) to light. Phil. Trans. Roy. Soc. London. 147:145–181.
- ↑ Buffat, P.H., and J.P. Burrel. 1976. Size effect on the melting temperature of gold particles. Physical Review A. 13(6):2287–2298. Retrieved November 16, 2008.
- ↑ Agam, and Guo. 2007. Electron beam modification of polymer nanospheres. Journal of Nanoscience and Nanotechnology. 7(10):3615-3619. Digital object identifier (DOI): 10.1166/jnn.2007.814 .
- ↑ Choy, J.H., E.S. Jang, J.H. Won, J.H. Chung, D.J. Jang, and Y.W. Kim. 2004. Hydrothermal route to ZnO nanocoral reefs and nanofibers. Appl. Phys. Lett. 84:287.
- ↑ Sun, Yugang, and Younan Xia. 2002. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science. 298:2176. Digital object identifier (DOI): 10.1126/science.1077229 .
- ↑ Murphy, Catherine. 2002. Nanocubes and Nanoboxes. Science 298:2139. Digital object identifier (DOI): 10.1126/science.1080007 .
- ↑ Mnyusiwalla, Anisa, Abdallah S. Daar, and Peter A. Singer. 2003. 'Mind the gap': science and ethics in nanotechnology. Nanotechnology. 14:R9-R13. Template:Doi:10.1088/0957-4484/14/3/201.
- ↑ Ying, Jackie. 2001. Nanostructured Materials. San Diego, CA: Academic Press. ISBN 9780120085279.
- ↑ Nanotechnologies: 6. What are potential harmful effects of nanoparticles? ec.europa.eu. Retrieved November 15, 2008.
- ↑ Davidson, Keay. 2006. FDA urged to limit nanoparticle use in cosmetics and sunscreens. San Francisco Chronicle. Retrieved November 15, 2008.
- ↑ Satariano, Adam. 2008. study Pollution Particles Lead to Higher Heart Attack Risk (Update1). Bloomberg.com. Retrieved November 15, 2008.
- ↑ Korzeniewski, Jeremy. 2007. Silicon nanoparticle film can increase solar cell performance. Autoblog Green. Retrieved November 15, 2008.
ReferencesISBN links support NWE through referral fees
External links
- International Liposome Society
- Textiles Nanotechnology Laboratory at Cornell University
- Assessing health risks of nanoparticles summary by GreenFacts of the European Commission SCENIHR assessment
- IOP.org Article
- Nano Structured Material
- Nanoparticles Used In Solar Energy Conversion (ScienceDaily).
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.