Difference between revisions of "Maltose" - New World Encyclopedia
(New page: {{Claimed}}) |
|||
| Line 1: | Line 1: | ||
{{Claimed}} | {{Claimed}} | ||
| + | <!-- Here is a table of data; skip past it to edit the text. —> | ||
| + | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
| + | ! {{chembox header}}| '''{{{name|{{PAGENAME}}}}}'''<ref>''Merck Index'', 11th Edition, '''5595'''.</ref> | ||
| + | |- | ||
| + | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:Maltose structure.svg|199px]] | ||
| + | |- | ||
| + | | [[IUPAC nomenclature|Chemical name]] | ||
| + | | {{{IUPAC|4-O-α-<small>D</small>-Glucopyranosyl-<small>D</small>-glucose}}} | ||
| + | |- | ||
| + | | Other names | ||
| + | | Maltose<br>Malt sugar<br>Maltobiose | ||
| + | |- | ||
| + | | [[Chemical formula]] | ||
| + | | {{{formula|C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>}}} | ||
| + | |- | ||
| + | | [[Molecular mass]] | ||
| + | | {{{mol_mass|342.1162}}} g/mol | ||
| + | |- | ||
| + | | [[CAS registry number|CAS number]] | ||
| + | | [{{{CAS|69-79-4}}}] | ||
| + | |- | ||
| + | | [[Density]] | ||
| + | | {{{density|1.54}}} g/cm<sup>3</sup> <ref name="MSDS">[http://www.bme.utexas.edu/ugrad/UGLab/resources/MSDS/maltose.pdf MSDS for maltose monohydrate]</ref> | ||
| + | |- | ||
| + | | Solubility | ||
| + | | 1.080 g/ml (20 °C) in water<ref name="MSDS"/> | ||
| + | |- | ||
| + | | [[Melting point]] | ||
| + | | {{{melting_point|102-103}}} °C (monohydrate) | ||
| + | |- | ||
| + | | [[Boiling point]] | ||
| + | | {{{boiling_point|N/A}}} | ||
| + | |- | ||
| + | | [[Simplified molecular input line entry specification|SMILES]] | ||
| + | | {{{SMILES|<small>OC[C@H]1O[C@H](O[C@H<br>]2[C@H](O)[C@@H](O)C(O)O<br>[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O</small>}}} | ||
| + | |- | ||
| + | | {{chembox header}} | <small>[[wikipedia:Chemical infobox|Disclaimer and references]]</small> | ||
| + | |- | ||
| + | |} | ||
| + | |||
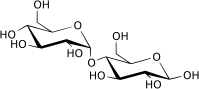
| + | '''Maltose''', or malt sugar, is a [[disaccharide]] formed from two units of [[glucose]] joined with an α(1→4) linkage. It is the second member of an important biochemical series of glucose chains. The addition of another glucose unit yields [[maltotriose]], Further additions will produce [[dextrin]]s, also called maltodextrins, and eventually [starch]. | ||
| + | |||
| + | Maltose can be broken down into two glucose molecules by [[hydrolysis]]. In living [[organism]]s, the [[enzyme]] [[maltase]] can achieve this very rapidly. In the laboratory, heating with a [[strong acid]] for several minutes will produce the same result. | ||
| + | |||
| + | The production of maltose in [[germinating]] [[cereals]], such as [[barley]], is an important part of the [[brewing]] process. When barley is [[malt]]ed, it is brought into a condition in which the concentration of maltose has been maximized. Metabolism of maltose by yeast during fermentation then leads to the production of [[ethanol]] and [[carbon dioxide]]. | ||
| + | |||
| + | == See also == | ||
| + | * [[Sugar]] | ||
| + | * [[Disaccharide]] | ||
| + | * [[Glucose]] | ||
| + | * [[Fructose]] | ||
| + | * [[Maltase]] | ||
| + | * [[Iso maltase]] | ||
| + | |||
| + | ==References== | ||
| + | <references/> | ||
| + | |||
| + | ==External links== | ||
| + | *[http://www.che.utoledo.edu/nadarajah/webpages/mbp.htm ''Detailed Views of'' Maltose Binding Protein] | ||
| + | *[http://www.elmhurst.edu/~chm/vchembook/546maltose.html ''Elmhurst College Virtual Chembook''] | ||
| + | {{ChemicalSources}} | ||
| + | |||
| + | {{Carbohydrates}} | ||
| + | |||
| + | [[Category:Disaccharides]] | ||
| + | [[Category:Sweeteners]] | ||
| + | {{credit|134045744}} | ||
| + | [[Category:Life sciences]] | ||
Revision as of 16:03, 15 June 2007
| Maltose[1] | |
|---|---|
| |
| Chemical name | 4-O-α-D-Glucopyranosyl-D-glucose |
| Other names | Maltose Malt sugar Maltobiose |
| Chemical formula | C12H22O11 |
| Molecular mass | 342.1162 g/mol |
| CAS number | [69-79-4] |
| Density | 1.54 g/cm3 [2] |
| Solubility | 1.080 g/ml (20 °C) in water[2] |
| Melting point | 102-103 °C (monohydrate) |
| Boiling point | N/A |
| SMILES | OC[C@H]1O[C@H](O[C@H ]2[C@H](O)[C@@H](O)C(O)O [C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O |
| Disclaimer and references | |
Maltose, or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) linkage. It is the second member of an important biochemical series of glucose chains. The addition of another glucose unit yields maltotriose, Further additions will produce dextrins, also called maltodextrins, and eventually [starch].
Maltose can be broken down into two glucose molecules by hydrolysis. In living organisms, the enzyme maltase can achieve this very rapidly. In the laboratory, heating with a strong acid for several minutes will produce the same result.
The production of maltose in germinating cereals, such as barley, is an important part of the brewing process. When barley is malted, it is brought into a condition in which the concentration of maltose has been maximized. Metabolism of maltose by yeast during fermentation then leads to the production of ethanol and carbon dioxide.
See also
- Sugar
- Disaccharide
- Glucose
- Fructose
- Maltase
- Iso maltase
ReferencesISBN links support NWE through referral fees
- ↑ Merck Index, 11th Edition, 5595.
- ↑ 2.0 2.1 MSDS for maltose monohydrate
External links
Template:ChemicalSources
| |||||||||||||||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.